Abstract
Objectives of this study were to establish a leukemia mouse model in the Balb/c mouse based upon the A20 cell line (murine B-lymphoma/leukemia cell line, H-2d). Here we demonstrate for the first time that A20 cells were infiltrated into tissue and bone marrow, thereby evaluate the feasibility of using A20 leukemic cells as a leukemia model. In the study, changes of behavior, survival rate and histological changes of major organs after intravenous injection of A20 cells (1×105, 1×106 or 1×107) into Balb/c mice were observed. After inoculation of 1×106 cells, animals survived up to 38.3 days, although there were no significant correlation between the number of injected cells and life-span. At 21 and 28 days postinjection, both hematoxylin-eosin and CD45R immunohistochemical stains showed diffuse large B-cell lymphoma in the liver. FACS analysis was performed after injection of fluorescent nanomaterial (MNPs@SiO2 RITC)-labeled A20 cells. The labeled A20 cells were detected in bone marrow from 6 hours post-inoculation, indicative of the cellular infiltration. This is the first study that demonstrated the invasion of A20 cells into the bone marrow of Balb/c model using A20 cells. With the occurrence of systemic lesions following metastasis of the cells into lymph nodes and neighboring tissues via bone marrow infiltration, it is suggested that the A20 cell-inoculated Balb/c miouse could be an animal model of acute lymphocytic leukemia.
References
Benjamin, R., Khwaja, A., Singh, N., McIntosh, J., Meager, A., Wadhwa, M., Streck, C., Ng, C., Davidoff, A.M. and Nathwani, A.C. (. 2007. ). Continuous delivery of human type I interferons (alpha/beta) has significant activity against acute myeloid leukemia cells in vitro and in a xenograft model. Blood. 109(3):1244–1247.
Bonnet, D., Bhatia, M., Wang, J.C., Kapp, U. and Dick, J.E. (. 1999. ). Cytokine treatment or accessory cells are required to initiate engraftment of purified primitive human hematopoietic cells transplanted at limiting doses into NOD/SCID mice. Bone Marrow Transplant. 23(3):203–209.
Caretto, P., Forni, M., d'Orazi, G., Scarpa, S., Feraiorni, P., Jemma, C., Modesti, A., Ferrarini, M., Roncella, S. and Foà, R. (. 1989. ). Xenotransplantation in immunosuppressed nude mice of human solid tumors and acute leukemias directly from patients or in vitro cell lines. Ric. Clin. Lab. 19(3):231–243.
Drexler, H.G., Matsuo, A.Y. and MacLeod, R.A. (. 2000. ). Continuous hematopoietic cell lines as model systems for leukemia-lymphoma research. Leuk. Res. 24(11):881–911.
Fingert, H.J., Chen, Z., Mizrahi, N., Gajewski, W.H., Bamberg, M.P. and Kradin, R.L. (. 1987. ). Rapid growth of human cancer cells in a mouse model with fibrin clot subrenal capsule assay. Cancer Res. 47(14):3824–3829.
Fu, X.Y., Theodorescu, D., Kerbel, R.S. and Hoffman, R.M. (. 1991. ). Extensive multiorgan metastasis following orthotopic onplantation of histologically-intact human bladder carcinoma tissue in nude mice. Int. J. Cancer. 49(6):938–939.
Gahrton G. (. 1983. ). Treatment of acute leukemia–advances in chemotherapy, immunotherapy, and bone marrow transplantation. Adv. Cancer Res. 40:255–329.
Giovanella, B.C. and Stehlin, JS. (. 1973. ). Heterotransplantation of human malignant tumors in “nude” thymusless mice. I. Breeding and maintenance of “nude” mice. J. Natl. Cancer Inst. 51(2):615–619.
Giovanella, B.C., Stehlin, J.S. and Williams, L.J. Jr. (. 1974. ). Heterotransplantation of human malignant tumors in “nude” thymusless mice. II. Malignant tumors induced by injection of cell cultures derived from human solid tumors. J. Natl. Cancer Inst. 52(3):921–930.
Glass, B., Uharek, L., Zeis, M., Loeffler, H., Mueller-Ruchholtz, W. and Gassmann, W. (. 1996. ). Graft-versus-leukaemia activity can be predicted by natural cytotoxicity against leukaemia cells. Br. J. Haematol. 93(2):412–420.
Goldman, J.M. (. 1987. ). Prospects for cure in leukaemia. J. Clin. Pathol. 40(9):985–994.
Hawkins, B.L., Heniford, B.W., Ackermann, D.M., Leonberger, M., Martinez, S.A. and Hendler, F.J. (. 1994. ). 4NQO carcinogenesis: A mouse model of oral cavity squamous cell carcinoma. Head Neck. 16(5):424–432.
Huang, S.M. and Harari, P.M. (. 2000. ). Modulation of radiation response after epidermal growth factor recept or blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin. Cancer Res. 6(6):2166–2174.
Huang, S.M., Li, J., Armstrong, E.A. and Harari, P.M. (. 2002. ). Modulation of radiation response and tumor-induced angiogenesis after epidermal growth factor receptor inhibition by ZD1839 (Iressa). Cancer Res. 62(15):4300–4306.
Janssen, J.W., Steenvoorden, A.C., Losekoot, M. and Bartram, C.R. (. 1987. ). Novel transforming sequences in human acute myelocytic leukemia cell lines. Oncogene. 1(2):175–179.
Killion, J.J., Radinsky, R. and Fidler, I.J. (. 1998. ). Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer metastasis Rev. 17(3):279–284.
Kim, J.S., Yoon, T.J., Yu, K.N., Kim, B.G., Park, S.J., Kim, H.W., Lee, K.H., Park, S.B., Lee, J.K. and Cho, M.H. (. 2006. ). Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 89(1):338–347.
Kim, J.S., Yoon, T.J., Yu, K.N., Noh, M.S., Woo, M., Kim, B.G., Lee, K.H., Sohn, B.H., Park, S.B., Lee, J.K. and Cho, M.H. (. 2006. ). Cellular uptake of magnetic nanoparticle is mediated through energy-dependent endocytosis in A549 cells. J.Vet. Sci. 7(4):321–326.
Kiser, M., McCubrey, J.A., Steelman, L.S., Shelton, J.G., Ramage, J., Alexander, R.L., Kucera, G.L., Pettenati, M., Willingham, M.C., Miller, M.S. and Frankel, A.E. (. 2001. ). Oncogene-dependent engraftment of human myeloid leukemia cells in immunosuppressed mice. Leukemia. 15(5):814–818.
Krishnaraju, K., Hoffman, B. and Liebermann, D.A. (. 1998. ). The zinc finger transcription factor Egr-1 activates macrophage differentiation in M1 myeloblastic leukemia cells. Blood. 92(6):1957–1966.
Liu, J., Li, Y., Tang, L.J., Zhang, G.P. and Hu, W.X. (. 2007. ). Treatment of lycorine on SCID mice model with human APL cells. Biomed. Pharmacother. 61(4):229–234.
Lumkul, R., Gorin, N.C., Malehorn, M.T., Hoehn, G.T., Zheng, R., Baldwin, B., Small, D., Gore, S., Smith, D., Meltzer, P.S. and Civin, C.I. (. 2002. ). Human AML cells in NOD/SCID mice: engraftment potential and gene expression. Leukemia. 16(9):1818–1826.
Machado, E.A., Gerard, D.A., Lozzio, C.B., Lozzio, B.B., Mitchell, J.R. and Golde, D.W. (. 1984. ). Proliferation and differentiation of human myeloid leukemic cells in immunodeficient mice: electron microscopy and cytochemistry. Blood. 63(5):1015–1022.
Marie, J.P. (. 2001. ). Drug resistance in hematologic malignancies. Curr. Opin. Oncol. 13(6):463–469.
McCormack, E., Bruserud, O. and Gjertsen, B.T. (. 2005. ). Animal models of acute myelogenous leukaemia–development, application and future Perspectives. Leukemia. 19(5):687–706.
Nara, N. and Miyamoto, T. (. 1982. ). Direct and serial transplantation of human acute myeloid leukaemia into nude mice. Br. J. Cancer. 45(5):778–782.
Park, K.S., Tae, J., Choi, B., Kim, Y.S., Moon, C., Kim, S.H., Lee, H.S., Kim, J., Kim, J., Park, J., Lee, J.H., Lee, J.E., Joh, J.W. and Kim, S. (. 2010. ). Characterization, in vitro cytotoxicity assessment, and in vivo visualization of multimodal, RITC-labeled, silica-coated magnetic nanoparticles for labeling human cord blood-derived mesenchymal stem cells. Nanomedicine. 6(2):263–276.
Passineau, M.J., Siegal, G.P., Everts, M., Pereboev, A., Jhala, D., Wang, M., Zhu, Z.B., Park, S.K., Curiel, D.T. and Nelson, G.M. (. 2005. ). The natural history of a novel, systemic, disseminated model of syngeneic mouse B-cell lymphoma. Leuk. Lymphoma. 46(11):1627–1638.
Perentesis, J.P., Gunther, R., Waurzyniak, B., Yanishevski, Y., Myers, D.E., Ek, O., Messinger, Y., Shao, Y., Chelstrom, L.M., Schneider, E., Evans, W.E. and Uckun, F.M. (. 1997. ). In vivo biotherapy of HL-60 myeloid leukaemia with a genetically engineered recombinant fusion toxin directed against the human granulocyte macrophage colonystimulating factor receptor. Clin. Cancer Res. 3:2217–2227.
Pieters, R. and Carroll, W.L. (. 2010. ). Biology and treatment of acute lymphoblastic leukemia. Hematol. Oncol. Clin. North. Am. 24(1):1–18.
Ratajczak, M.Z., Kant, J.A., Luger, S.M., Hijiya, N., Zhang, J., Zon, G. and Gewirtz, A.M. (. 1992. ). In vivo treatment of human leukemia in a scid mouse model with c-myb antisense oligodeoxynucleotides. Proc. Natl. Acad. Sci. U S A. 89(24):11823–11827.
Rombouts, W.J., Martens, A.C. and Ploemacher, R.E. (. 2000. ). Identification of variables determining the engraftment potential of human acute myeloid leukemia in the immunodeficient NOD/SCID human chimera model. Leukemia. 14(5):889–897.
Scheper, M.A., Nikitakis, N.G., Chaisuparat, R., Montaner, S. and Sauk, J.J. (. 2007. ). Sulindac induces apoptosis and inhibits tumor growth in vivo in head and neck Squamous cell carcinoma. Neoplasia. 9(3):192–199.
Seif, A.E., Barrett, D.M., Milone, M., Brown, V.I., Grupp, S.A. and Reid, G.S. (. 2009. ). Long-term protection from syngeneic acute lymphoblastic leukemia by CpG ODN-mediated stimulation of innate and adaptive immune responses. Blood. 114(12):2459–2466.
Shultz, L.D., Schweitzer, P.A., Christianson, S.W., Gott, B., Schweitzer, I.B., Tennent, B., McKenna, S. Mobraaten, L., Rajan, T.V., Greiner, D.L. and Leite, E.H. (. 1995. ). Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154(1):180–191.
Tomkinson, B., Bendele, R., Giles, F.J., Brown, E., Gray, A., Hart, K., LeRay, J.D., Meyer, D., Pelanne, M. and Emerson, D.L. (. 2003. ). OSI-211, a novel liposomal topoisomerase I inhibitor, is active in SCID mouse models of human AML and ALL. Leuk. Res. 27(11):1039–1050.
Umeda, M., Komatsubara, H., Nishimatsu, N., Oku, N., Shibuya, Y., Yokoo, S. and Komori, T. (. 2002. ). Establishment and characterization of a human adenoid cystic carcinoma line of the salivary gland which is serially transplantable and spontaneously metastasis to the lung in nude mice. Oral Oncol. 38(1):30–34.
Wasserman, R., Zeng, X.X. and Hardy, R.R. (. 1998. ). The evolution of B precursor leukemia in the E-mu-ret mouse. Blood. 92(1):273–282.
Watanabe, S., Shimosato, Y., Kuroki, M., Sato, Y. and Nakajima, T. (. 1980. ). Transplantability of human lymphoid cell line, lymphoma, and leukemia in splenectomized and/or irradiated nude mice. Cancer Res. 40(7):2588–2595.
Xu, Y. and Scheinberg, D.A. (. 1995. ). Elimination of human leukemia by monoclonal antibodies in an athymic nude mouse leukemia model. Clin. Cancer Res. 1(10):1179–1187.
Zeis, M., Uharek, L., Hartung, G., Glass, B., Steinmann, J. and Schmitz, N. (. 2001. ). Graft-vs-leukemia activity and graft-vs-host disease induced by allogeneic Th1- and Th2-type CD4+ T cells in mice. Hematol. J. 2(2):136–144.
Zeis, M., Uharek, L., Glass, B., Steinmann, J., Dreger, P., Gassmann, W. and Schmitz, N. (. 1997. ). Allogeneic MHC-mismatched activated natural killer cells administered after bone marrow transplantation provide a strong graft-versus-leukaemia effect in mice. Br. J. Haematol. 96(4):757–761.
Zeng, X.X., Zhang, H., Hardy, R.R. and Wasserman, R. (. 1998. ). The fetal origin of B-precursor leukemia in the E-mu-ret mouse. Blood. 92(10):3529–3536.
Figure 1.
Morphology and CD45R expression of A20 cell line, showing features immediately (A) and 48 hours after seeding (B) (scale bar: 50 µm), Wright's staining (C) (scale bar: 100 µm), and CD45R expression on the cell surface (D).
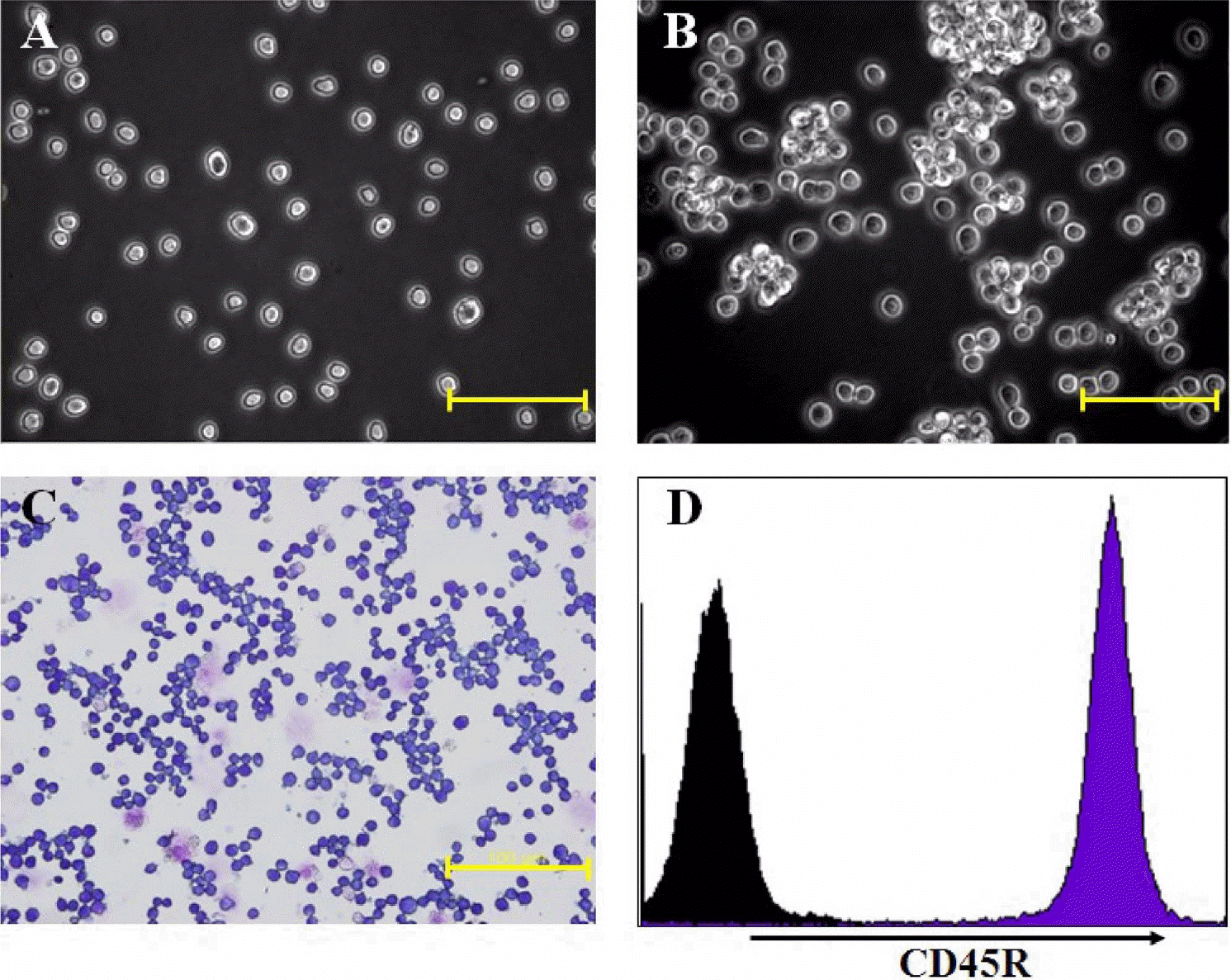
Figure 2.
Survival rate of A20 cell-transplanted Balb/c mice. Three different numbers (1×105, 1×106 or 1×107 cells) of A20 leukemia cells were intravenously injected into Balb/c mice. Each group consisted of 6–13 mice.
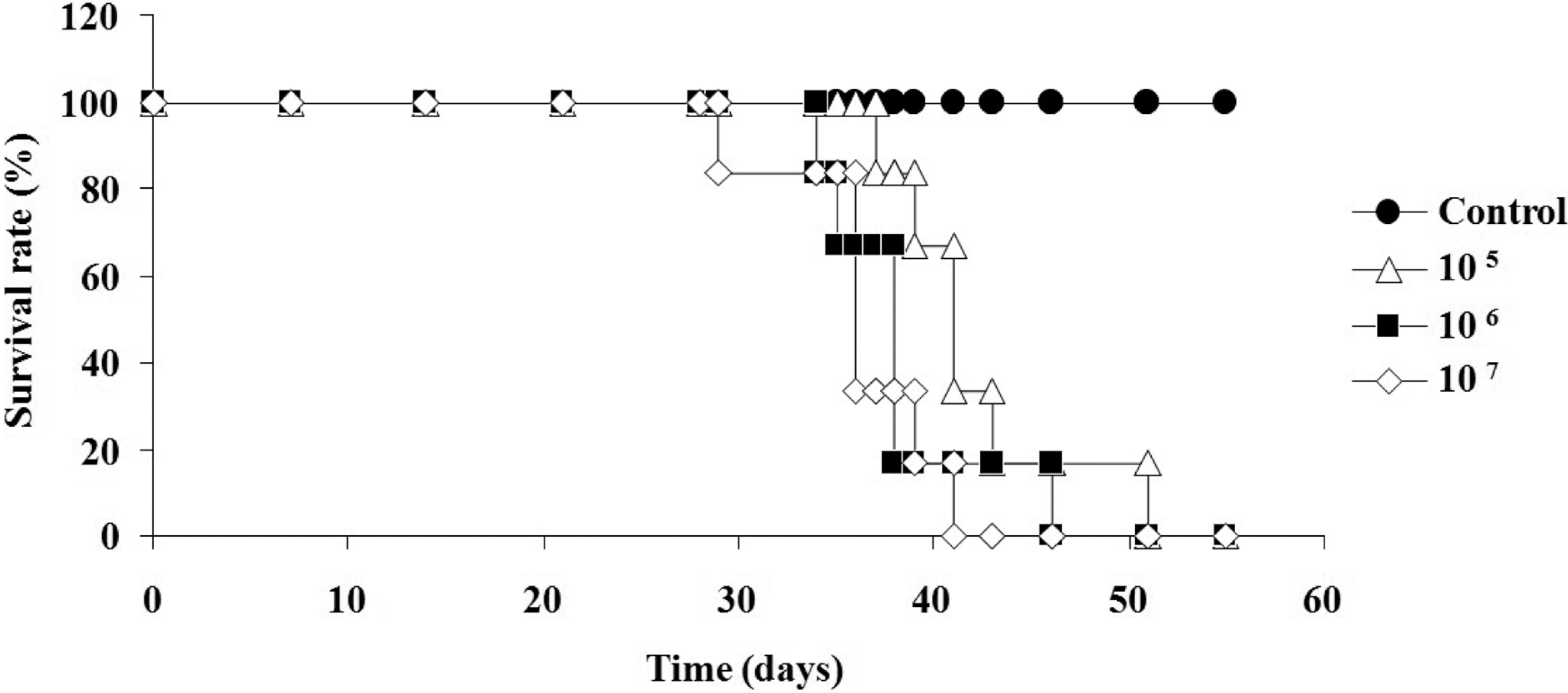
Figure 3.
Comparison of the livers of control and A20 cell-inoculated mice. Livers were removed at 28 days post-injection. The surface of control liver is smooth and soft, while distinct nodules are visible on the A20 cell-transplanted mice (A-D). Histological examination showed diffuse or nodular infiltration of lymphoma cells in the liver parenchyma of A20 cell-transplanted mice. The infiltrated A20 cells were confirmed by H&E (E-G) and immunohistochemical staining on CD45R (H-K). Scale bar: 500 µm.
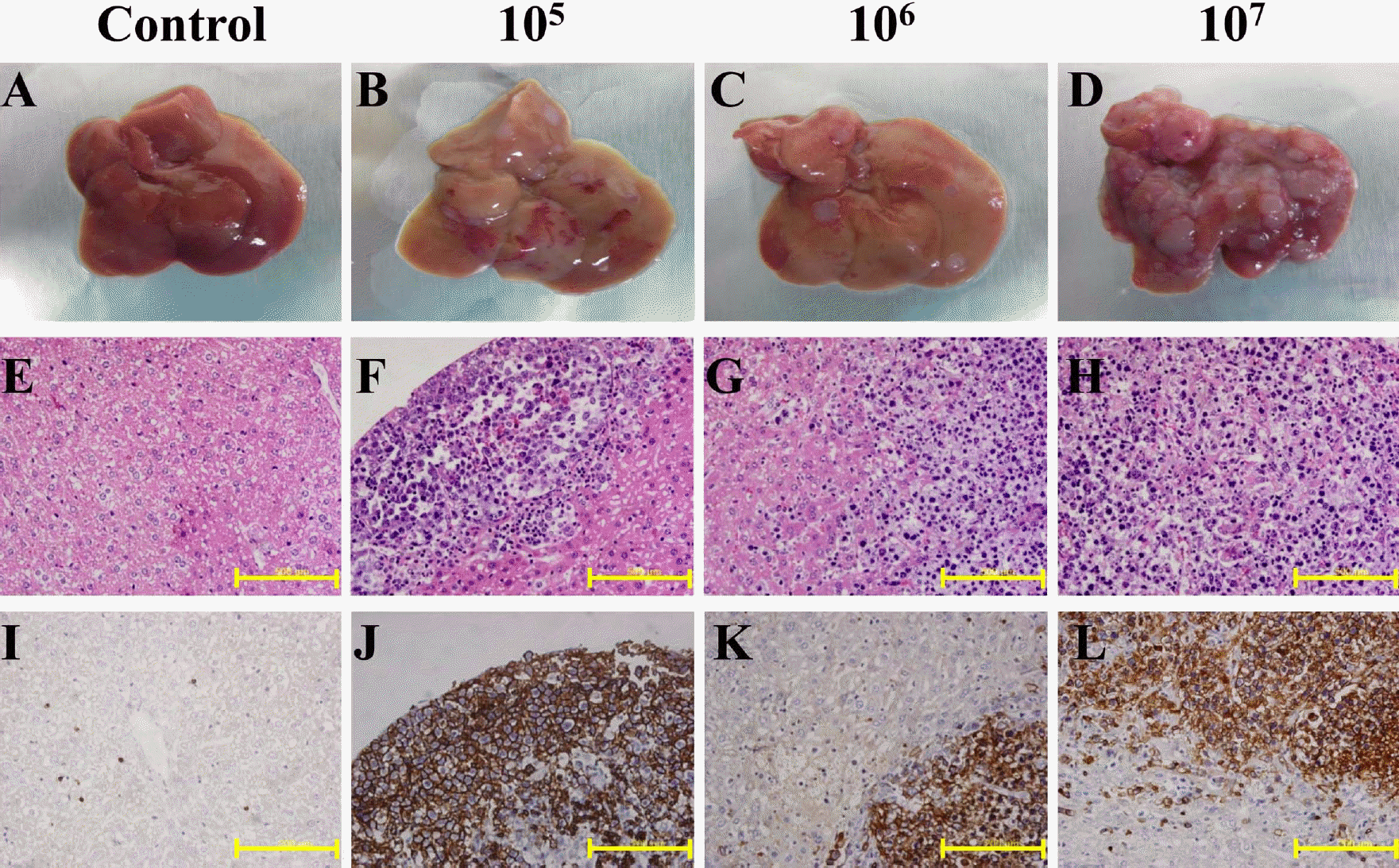
Figure 4.
Detection of fluorescent nanoparticle (MNPs@SiO2 RITC)-labeled A20 cells in vitro and in vivo. RITC signals in the labeled cells were detected by fluorescence microscopy (A) and FACS (B). In vivo, the infiltrations of A20 cells in PBMC (C) and bone marrow (D) were analysis by FACS. Data at every time point in each group consisted of 3 mice.
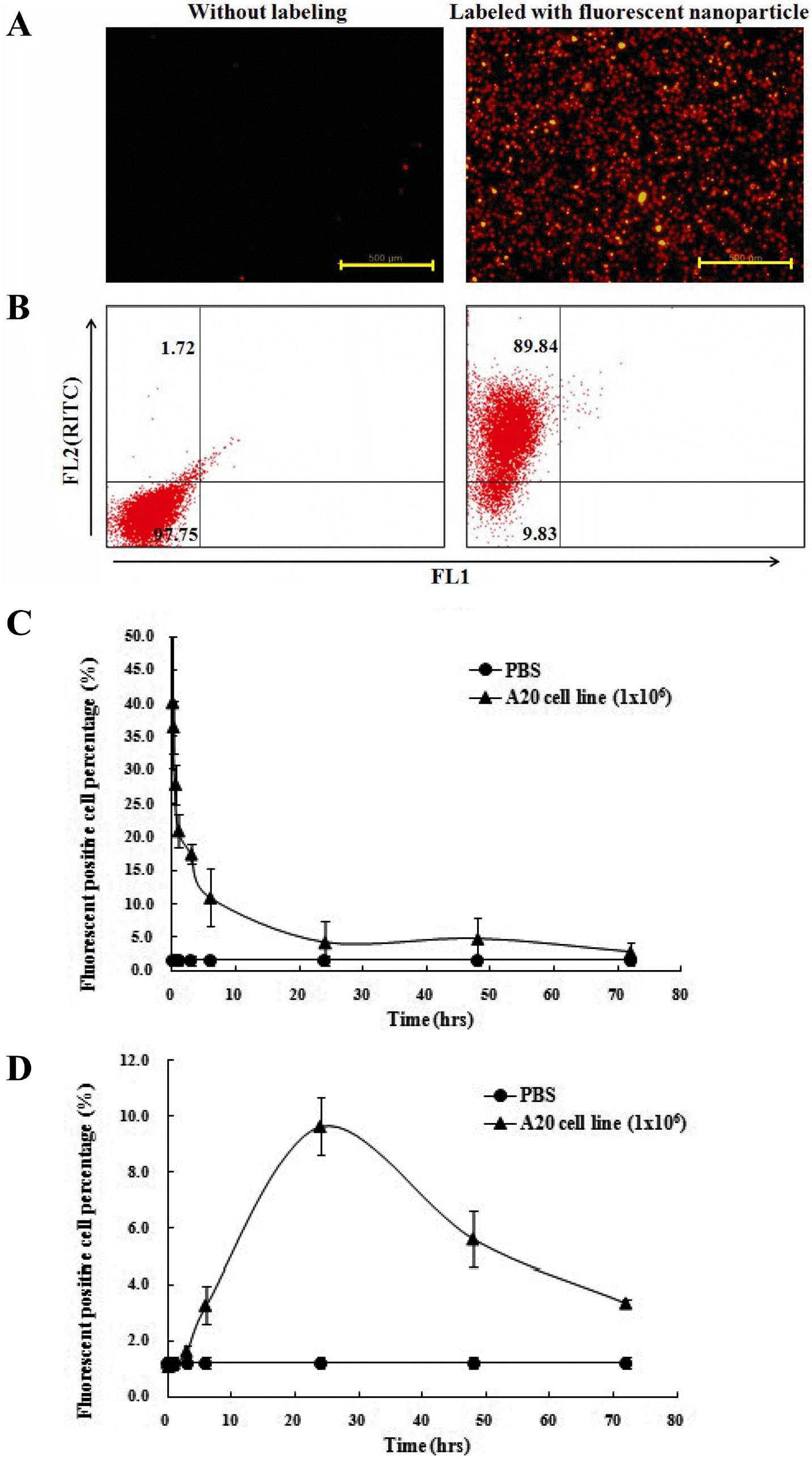
Table 1.
Body and tissue weights of A20 cell-transplanted Balb/c mice
| Treatment (cells) | Survival (days) | Body weight (g) | Liver weight (g) | Spleen weight (g) | ||
|---|---|---|---|---|---|---|
| 0 week | 3 week | 4 week | 4 week | 4 week | ||
| PBS | ∞ | 100.0±4.5 | 108.5±7.1 | 111.0±5.4 | 1.351±0.051 | 0.074±0.006 |
| 1x105 | 42.5±6.2 | 100.0±5.4 | 107.5±6.3 | 110.9±7.6 | 1.374±0.021 | 0.078±0.005 |
| 1x106 | 38.3±5.4 | 100.0±3.7 | 104.1±6.4 | 110.1±4.1 | ∗1.590±0.082∗ | 0.087±0.010 |
| 1x107 | 36.3±5.3 | 100.0±1.6 | 107.6±3.1 | 103.4±4.7 | ∗1.985±0.167∗ | ∗0.134±0.121∗ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download