Abstract
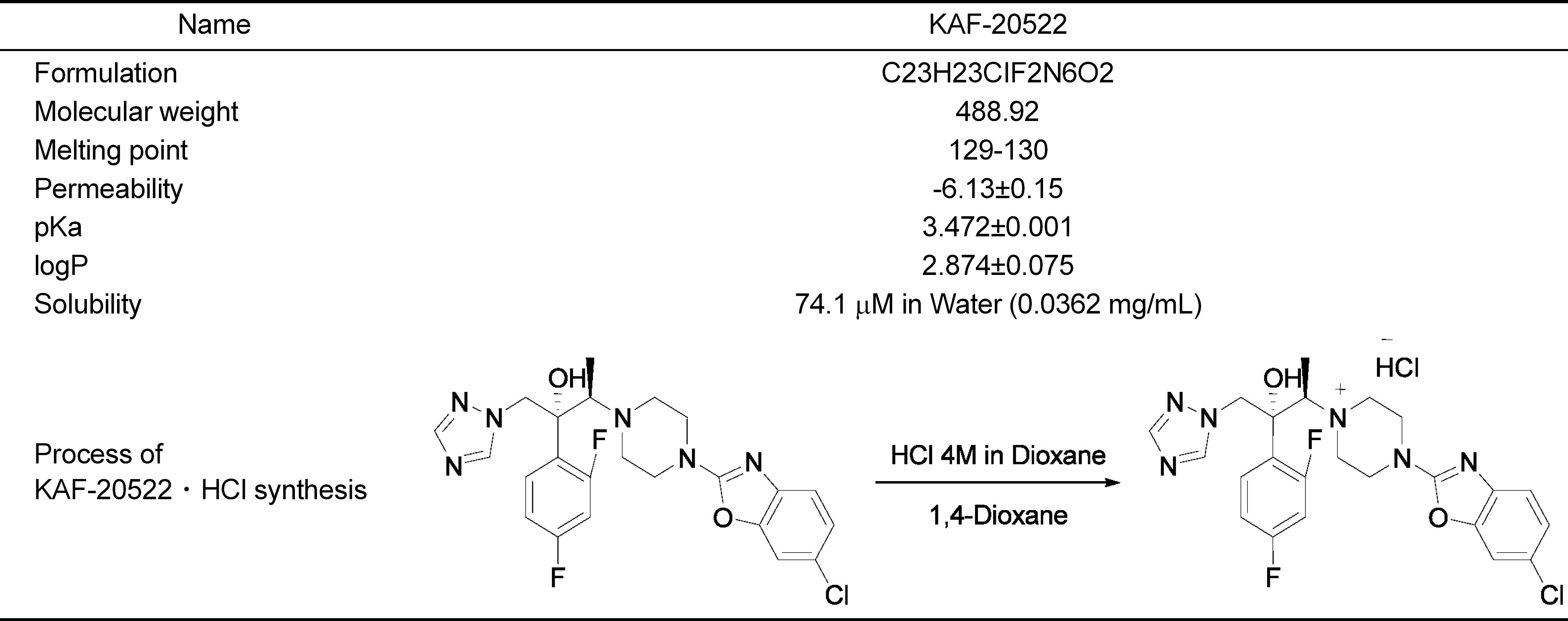
Recent researches on clinically used triazole antifungal reagents are focused on their pharmacokinetic disadvantage which increases the probability of inducing adverse effects in patients. For this point, in the present laboratory, Chemon Inc., has investigated new antifungal reactive compounds, KAF-200522 and its chloride form, KAF-200522 · HCl, which has a modified triazole structure. Pharmacokinetic data were measured with LC-MS/MS in male mice which were orally treated with the above compounds at 10 mg/ kg. Tmax and t1/2 of KAF-200522 · HCl were comparable to KAF-200522, but AUC and Cmax were 1.4 and 1.6 times higher than those of KAF-200522, respectively. In beagle dogs, AUC and Cmax of KAF-200522 · HCl were 2.7 and 1.4 times higher than those of KAF-200522, and t1/2 was 3.5 times higher than that of KAF-200522. Moreover, in beagle dogs, the oral bioavailability value of KAF-200522 · HCl was revealed as 31.0% to contrast to 6.2% of KAF-200522. In 1-week repeated oral treatment toxicity study of KAF-200522 in male rats, inhibition of body weight gain was observed in 120 mg/kg treatment group, and loss of body weight was observed in 600 mg/kg treatment group. In the toxicokinetic study of KAF-200522, no accumulation after the systemic exposure was observed. In conclusion, as to the new antifungal drug development, KAF-200522 · HCl was considered to be advantageous in pharmacokinetic characteristics compared to KAF-200522.
Go to : 
References
Abruzzo, G.K., Fromtling, R.A., Turnbull, T.A. and Giltinan, D.M. (. 1987. ). Effects of bifonazole, fluconazole, itraconazole, and terbinafine on the chemiluminescence response of immune cells. J. Antimicrob. Chemothrer. 20(1):61–68.
Arathoon, E.G. (. 2001. ). Clinical efficacy of echinocandin antifungals. Curr. Opin. Infect. Dis. 14(6):685–691.
Cornely, O.A., Ullmann, A.J. and Carthaus, M. (. 2003. ). Evidence based assessment of primary antifungal prophylaxis in patients with hematologic malignancies. Blood. 101:3365–3372.
Gartner, K., Buttner, D., Dohler, K., Friedel, R., Lindena, J. and Trautschold, I. (. 1980. ). Stress response of rats to handling and experimental procedures. Lab. Anim. 14:267–274.
Gimenia, C., Tuccinardi, C., Santilli, S., Mondello, F., Monaco, M., Cassone, A. and Martino, P. (. 2000. ). In vitro activity of fluconazole and voriconazole against isolates of Candida albicans from patients with haematological malignancies. J. Antimicrob. Chemother. 46(3):479–483.
Gupta, A.K., Sauder, D.N. and Shear, N.H. (. 1994. ). Antifungal agents: an overview. Part I. J. Am. Acad. Dermatol. 30:(. (5 Pt 1):),. 677–698.
Korea Food and Drug Administration. 2009. ). The Standards of Toxicity Study for Medicinal Products, p. 116, Korea Food and Drug Administration, Seoul. Korea Food and Drug Administration (2010) The Regulation for Review and Approval of Medicinal Products, p. 71, Korea Food and Drug Administration, Seoul. Hucker, H.B. (1970) Species differences in drug metabolism. Annu. Rev. Pharmacol. 10:99–118.
Humphrey, M.J., Jevons, S. and Tarbit, M.H. (. 1085. ). Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug in animals and humans. Antimicrob. Agents Chemother. 28(5):648–653.
O'Neill, P.J. and Kaufman, L.N. (. 1990. ). Effects of indwelling arterial catheters or physical restraint on food consumption and growth patterns of rats: advantages of noninvasive blood pressure measurement techniques. Lab. Anim. Sci. 40:641–643.
Plempel, M., Buchel, K.H., Bartmann, K. and Regel, E. (. 1974. ). Antimycotic properties of clotrimazole. Postgrad. Med. J. 50:11–12.
Rao, K.V. and Anderson, R.C. (. 1988. ). Long-term results and complications in renal transplant recipients. Observations in the second decade. Transplantation. 45(1):45–52.
Rex, J.H., Pfaller, M.A., Rinaldi, M.G., Polak, A. and Galgiani, J.N. (. 1993. ). Antifungal susceptibility testing. Clin. Microbiol. Rev. 6(4):367–381.
Saag, M.S. and Dismukes, W.E. (. 1988. ). Azole antifungal agents: emphasis on new triazoles. Antimicrob. Agents Chemother. 32:1–8.
Sanglard, D. and Odds, F.C. (. 2002. ). Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73–85.
Sheiner, L.B. and Steimer, J.L. (. 2000. ). Pharmacokinetic/pharmacodynamic modeling in drug development. Annu. Rev. Pharmacol. Toxicol. 40:67–95.
Walsh, T.J., Newman, K.R., Moody, M., Wharton, R.C. and Wade, J.C. (. 1986. ). Trichosporonosis in patients with neoplastic disease. Medicine. 65(4):268–279.
Walsh, T.J., Melcher, G.P., Rinaldi, M.G., Lecciones, J., McGough, D.A., Kelly, P., Lee, J., Callender, D., Rubin, M. and Pizzo, P.A. (. 1990. ). Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28(7):1616–1622.
Go to : 
Table 2.
Pharmacokinetic parameters of KAF-200522 and KAF-200522·HCl after a single oral administration of KAF-200522 and KAF-200522·HCl at 10 mg/kg to male mice (n=5, respectively)
| Compounds | t1/2 (hr) | Tmax (hr) | Cmax a) (ng/mL) | AUC (hr∗ng/mL) |
|---|---|---|---|---|
| KAF-200522 | 1.9 | 1.0 | 908±151 | 3687 |
| KAF-200522·HCl | 1.7 | 0.5 | 1488±2560 | 5150 |
Table 3.
Pharmacokinetic parameters for KAF-200522 from female beagle dogs after single administrations of KAF-200522 and KAF-200522·HCl at 10 mg/kg (KAF-200522; n=2, KAF-200522·HCl; n=4, respectively)
Table 4.
Body weight changes after 1 week repeated oral administrations of KAF-200522 in male rats (n=5)
| Dose (mg/kg/day) | Weights (g) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 3 | Day 6 | Gainsa) | ||
| TKb) | 182.89±3.71 | 197.29±5.18 | 202.53±6.35 | 226.23±8.67 | 43.35±5.10 | |
| 0 | Non-TKc) | 175.38±6.42 | 188.13±6.75 | 195.74±8.33 | 220.08±7.97 | 044.7±1.55 |
| Total | 179.88±5.84 | 193.63±7.07 | 199.82±7.17 | 223.77±8.05 | 43.89±3.76 | |
| TK | 180.87±8.13 | 0182.19±13.83 | 0188.56±13.34 | 0214.68±14.94 | 33.81±6.84 | |
| 24 | Non-TK | 184.19±0.07 | 197.74±1.85 | 204.14±3.76 | 225.68±2.04 | 41.49±1.97 |
| Total | 182.20±6.03 | 0188.41±13.00 | 0194.79±12.86 | 0219.08±12.20 | 36.88±6.48 | |
| TK | 182.83±5.56 | 181.82±6.71 | 187.88±9.31 | 0209.17±11.94 | 26.34±6.38 | |
| 120 | Non-TK | 177.94±6.02 | 184.66±9.02 | 0192.67±10.78 | 0212.56±10.29 | 34.62±4.27 |
| Total | 180.87±5.63 | 182.96±6.73 | 189.79±8.90 | 0.210.52±10.06∗ | ∗∗29.65±6.75∗∗ | |
| TK | 180.47±7.66 | 0157.28±12.73 | 0165.92±13.94 | 176.97±6.80 | 0–3.5±6.91 | |
| 600 | Non-TK | 180.18±0.54 | 160.51±2.94 | 172.61±1.99 | 180.73±0.18 | –0.55±0.72 |
| Total | 180.35±5.42 | 0.158.57±9.29∗∗ | ∗∗0168.60±10.56∗∗ | ∗∗178.47±5.23∗∗ | .0–1.88±5.38∗∗ | |
Table 5.
Mean toxicokinetic parameters for KAF-200522 from male Sprague-Dawley rats following 1-week repeated oral administrations of KAF-200522 (n=5)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download