Abstract
In this study, we investigated the in vitro antioxidative effects, antimicrobial activities and single oral dose toxicity of the extracts from Dansam-samultang to evaluate its use as a functional ingredient in cosmetics. In the antioxidative effect, the ethanol extract from Dansam-samultang (DSE) had higher antioxidant values of 92.0% at 1,000 µg/mL than that of water extract from Dansam-samultang (DSW, 86.0%) when evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity. The superoxide dismutase-like activity of DSW and DSE were 16.3% and 21.3% at 1,000 µg/mL in concentration, respectively. Xanthine oxidase inhibition activity of the DSE was higher 51.5% than that of the DSW (21.4%). This study was also undertaken to test the in vitro antimicrobial activity with the extracts of Dansam-samultang. In general, the DSE showed the significant antimicrobial activity against Staphylococcus aureus, Staphylococcus epidermiders and Escherichia coli. In single oral dose toxicity study, in vivo, there were no differences between control and treated groups in clinical signs, body weight gains, and gross finding. The results indicated that DSE did not show any toxic effects at 10 mL/kg in mice, and the LD50 of DSE was found to be higher than 10 mL/kg in this experiment. In conclusion, the extracts from Dansam-samultang may act as a natural subsistence for functional cosmetics.
References
Ahn, B.Y., Kim, D.G. and Chio, D.S. (. 1999. ). Antimultagenic effect of Tansen (Salvia miltiorrhiza Bunge). Korean. J. Appl. Microbiol. Biotechnol. 27(3):197–202.
Blois, M.S. (. 1958. ). Antioxidant determinaion by the use of stable free radical. Nature. 81:1198–1199.
Chang, B.Y., Oh, B.R., Sohn, D.H. and Kim, S.Y. (. 2008. ). Single Oral Toxicity Study on the Standardized Extract of Salvia miltiorrhiza, Kor. J. Pharmacogn. 39(4):352–356.
Choi, H.Y. and Han, Y.S. (. 2003. ). Isolation and identification of antimicrobial compound from Dansam (Salvia miltiorrhiza Bunge). J. Korean Soc. Food Sci. Nutr. 32(1):22–28.
De Philippis, R. and Vincenzni, M. (. 2001. ). Exopolysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 22(3):150–175.
Eom, J.N. and Kim, J.D. (. 2004. ). An empirical study on the oriental herbal cosmetics purchase behaviors in women in the Metropolitan area. J. Soc. Cosmet. Scientists Korea. 30(1):93–102.
Han, W.S. (. 2004. ). Isolation of Antimicrobial Compounds from Salvia miltorrhiza Bunge. Korean. J. Medicinal Crop Sci. 12(3):179–182.
Hase, K., Kasium, R., Basnet, P., Kadota, S. and Namba T. (. 1997. ). Preventive effect of lithlospermate B from Salvia miltiorrhiza on experimental hepatitis induced by carbon tetrachloride or D-galactosamine lipopolysaccharide. Planta Med. 63(1):22–26.
Hatano, T., Yasuhara, T., Fukida, T., Noro, T. and Okuda, T. (. 1989. ). Phenolic constituents of licoricell. Structures of licopyranocoumarin, licoarylcocumarin and glisoflavone, and inhibitory effects of licorice phenolics on xanthine oxidase. Chemical & Phamaceutical Bulletin. 37(11):3005–3009.
Higasi, G.S. (. 2000. ). Appraisement of antioxidative activity from vegetables. Jpn. J. Food Ind. 57(1):56–64.
Jeong, J.H., Wee, J.J., Shin, J.Y., Cho, J.H. and Jung, D.H. (. 2005. ). Antioxidative effect of crude saponin fraction prepared from culture product of basidiomycota cultured with fresh ginseng as substrate. Kor. J. Food Sci. Technol. 37(1):67–72.
Kang, H.S., Chung, H.Y., Byun, D.S. and Chio, J.S. (. 2005. ). Further isolation of antioxidative (+)-1-hydroxypinoresinol-1-O-β-D-glucoside from the rhizome of Salvia miltiorrhiza acts on peroxynitrite, total ROS and 1,1-diphenyl-2-picrylhydrazyl radical. Arch. Pharm. Res. 26(1):24–27.
Kang, S.A., Jang, K.H., Ahn, D.K. and Park, S.K. (. 2003. ). Differences of hematopoietic effects of Angelica gigas, A. sinensis and A. acutiloba extract on cyclophosphamide-induced anemic rats. Korean J Food Sci Technol. 35(6):1204–1208.
Kim, O.K., Chung, S.Y., Park, M.K., Rheu, H.M. and Yang, J.S. (. 1999. ). Anticancer activity of natural products including Salvia miltiorrhiza. J. Appl. Pharmacol. 7(1):29–33.
Lee, Y.J. and Lee, S.Y. (. 1992. ). Parmacognosy, pp. 131–137. Dong Myeung Sa, Seoul, Korea.
Marklund, S. and Marklund, G. (. 1974. ). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47:469–474.
Min, S.K. and Hong, C.H. (. 2007. ). Korean Traditional Medicines as Novel Drugs for Neuropsychiatric Disorders. Korean. J. Psychopharmacol. 18(1):5–17.
Mok, J.S., Kim, Y.M., Kim, S.H. and Chang, D.S. (. 1995. ). Antimicrobial property of the ethanol extract from Salvia miltiorrhiza. J. Food Hyg. Safety. 10(1):23–28.
Mok, J.S., Park, U.Y., Kim, Y.M. and Chang, D.S. (. 1994. ). Effects of solvents and extracting condition on the antimicrobial activity of Salviae miltiorrhizae Radix (Salvia miltiorrhiza) extract. J. Korean Soc. Food Nutr. 23(6):1001–1007.
Park, H.J., Lee, Y.J. and Keon, S.J. (. 2005. ). Pharmacognostical studies on the Dang Gui from Korea. Kor. J. Pharmacogn. 36(2):141–144.
Park, R.J., Kim, N.J., Lee, K.T. and Sed, S.H. (. 2001. ). Comparative studies on concentration of decursinol in plasma after oral administration of Angelica Gigantis Radix extract and combined use of decursin and Cnidii Rhizoma extract or Bupleuri Radix extract in rats. Kor. J. Pharmacogn. 32(1):72–78.
Park, U.J., Park, U.S., Im, M.H., Kim, H.J., An, J.S., Cho, J.Y. and Heo, B.G. (. 2008. ). Antibiotic activities of flower and leaf extracts from four species of white Lotus. J. Life Sci. & Nat. Res. 30(1):25–34.
Shon, Y.H., Kim, H.G. and Nam, K.S. (. 2003. ). Effect of Cnidii Rhizoma water extract on chemopreventive enzymes for hepato carcinoma. Kor. J. Pharmacogn. 34(4):297–302.
Stirpe, F. and Corte, E.D. (. 1969. ). The regulation of rat liver xanthine oxidase. J. Biol. Chem. 244(14):3855–3861.
Storch, J. and Ferber, E. (. 1988. ). Detergent-amplified chemiluminescence of lucigenin for determination of superoxide anion production by NADPH oxidase and xanthine oxidase. Analytical Biochemistry. 162(2):262–267.
Torel, J., Gillard, J. and Gillard, P. (. 1986. ). Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry. 25(2):383–385.
Figure 2.
Antioxidative effect of Dansam-samultang extracts on DPPH radical scavenging activity. DSW, Dansam-samultang water extracts; DSE, Dansam-samultang ethanol extracts. The values are means 3 replicates and those with different alphabet letters are significantly different at P<0.05.

Figure 3.
SOD-like activity of Dansam-samultang extracts. DSW, Dansam- samultang water extracts; DSE, Dansam-samultang ethanol extracts. The values are means 3 replicates and those with different alphabet letters are significantly different at P<0.05.
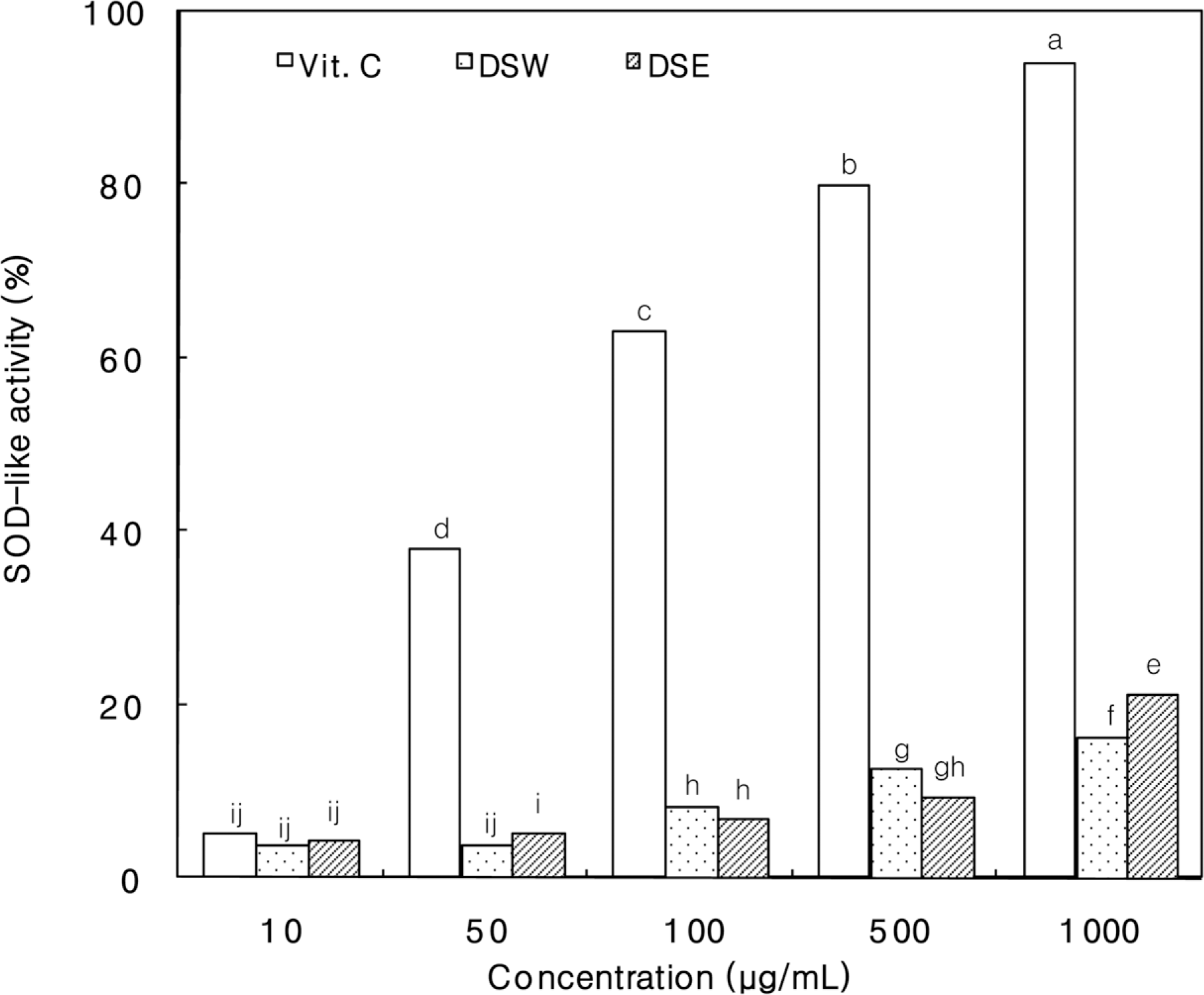
Figure 4.
Inhibition rate of Dansam-samultang extracts on xanthine oxidase. DSW, Dansam-samultang water extracts; DSE, Dansam-samultang ethanol extracts. The values are means 3 replicates and those with different alphabet letters are significantly different at P<0.05.
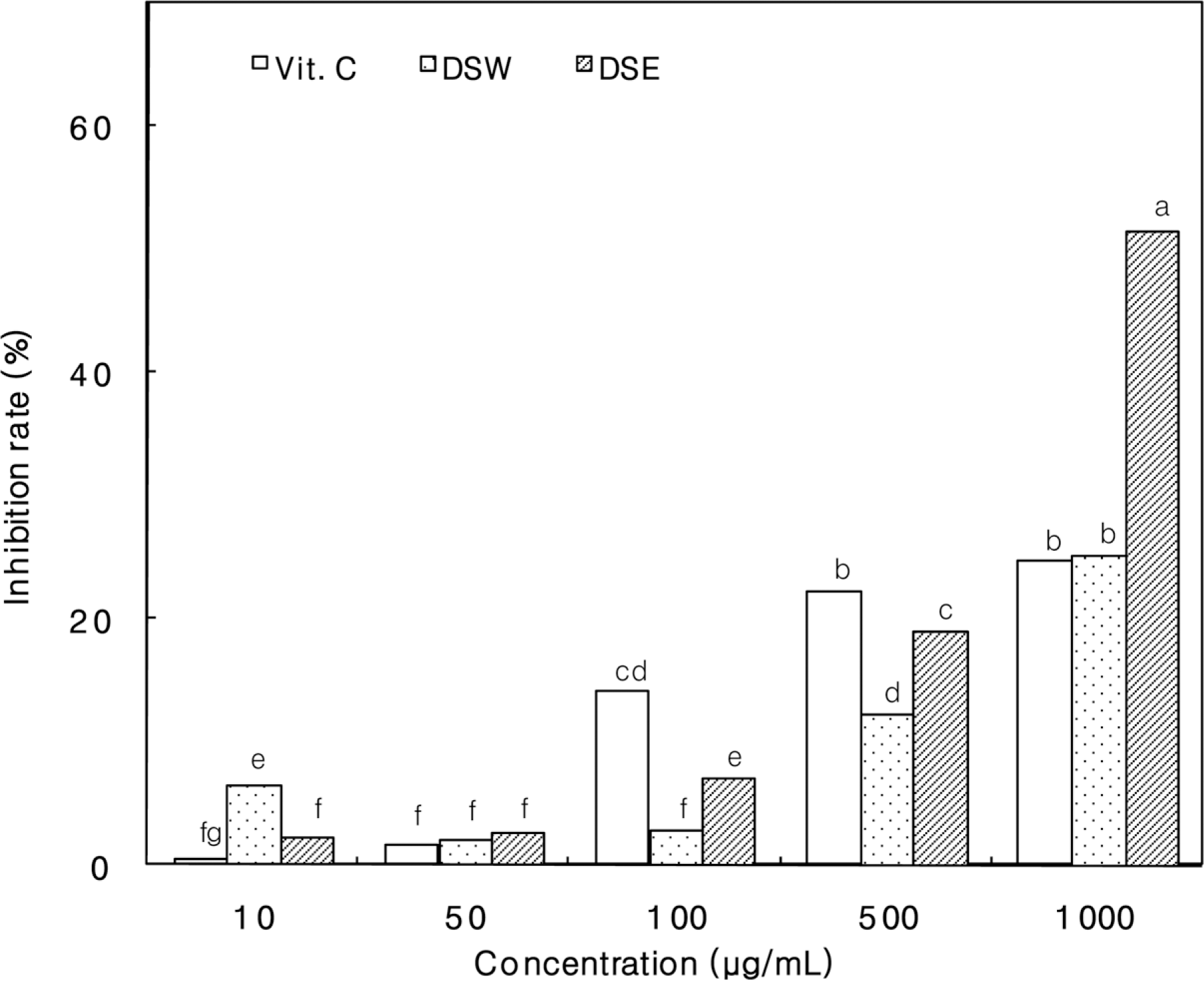
Table 1.
Composition of Dansam-samultang
Table 2.
Antimicrobial activity against three microorganisms by ethanol extracts from Dansam-samultang
| Strains | Clear zone on plate (mm)1 | |||
|---|---|---|---|---|
| Concentration (µg/mL) | ||||
| 50 | 100 | 200 | 400 | |
| Staphylococcus aureus KCTC 1621 | 8.0±0.0 | 8.0±0.0 | 14.7±0.3 | 17.3±0.1 |
| Staphylococcus epidermidis KCTC 1917 | 14.0±0.40 | 17.0±0.20 | 19.0±0.1 | 22.0±0.2 |
| Escherichia coli KCTC 1039 | 8.0±0.0 | 8.0±0.0 | 14.7±0.2 | 18.7±0.1 |
Table 3.
Mortality of male and female mice orally treated with Dansam-samultang ethanol extract (1%)
| Material | Sex | Injected volume (mL/kg) | Hours after treatment | Days after treatment | Final mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 3 | 7 | 14 | ||||
| Con1 | Male | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 |
| Female | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 | |
| DSE2 | Male | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 |
| Female | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 | |
Table 4.
Clinical signs of male and female mice orally treated with 10 mL/kg of Dansam-samultang ethanol extract (1%)
| Material | Sex | Clinical signs | Hours after treatment | Days after treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 3 | 7 | 14 | |||
| Con1 | Male | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Female | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| DSE2 | Male | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Female | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
Table 5.
Body weights of male and female mice orally treated with 10 mL/kg of Dansam-samultang ethanol extract (1%)
| Material | Sex | Number of animals | Days after treatment | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | |||
| Con1 | Male | 5 | 20.72±0.65 | 22.98±0.75 | 27.05±1.08 | 29.21±1.35 | 33.54±1.88 |
| Female | 5 | 19.83±0.52 | 20.84±0.95 | 25.09±1.25 | 28.11±1.54 | 30.51±2.43 | |
| DSE2 | Male | 5 | 21.18±0.65 | 21.88±0.93 | 26.72±1.38 | 28.15±1.61 | 32.34±1.89 |
| Female | 5 | 20.21±0.60 | 20.59±0.72 | 24.91±1.45 | 26.95±1.47 | 29.29±2.10 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download