Abstract
Brain damage resulting from perinatal cerebral hypoxia and ischemia is a major cause of acute mortality and neurological disabilities, including cerebral palsy (CP) and cognitive dysfunction. In order to establish an experimental hypoxia-ischemia (HI) model of CP for the screening of therapeutics, we operated bilateral common carotid artery ligation (BCAO) and monolateral carotid artery occlusion (MCAO), followed by 15 min of hypoxia (8% oxygen) in 4-day-old rats, and evaluated neurobehavioral disorders. After surgery, the survival rates of male and female BCAO rats were 33.3 and 7.1%, respectively, whereas 100% and 82.4% MCAO rats survived. In neurobehavioral performances, both male and female BCAO rats showed delayed achievement of righting reflex, in contrast to a negligible effect in MACO animals. However, both BCAO and MCAO rats exhibited impairment of cliff avoidance performances, although the physical dysfunction was more severe in BCAO than in MCAO. In global locomotor activity, MCAO rats also displayed decreased fast-moving time comparable BCAO animals, and increased resting and slow-moving times. In addition, MCAO rats showed marked learning and memory deficit in passive avoidance performances, similar to BCAO animals. From immunostaining analyses, severe degradation and loss of myelin basic proteins were observed in the brain of BCAO rats, in contrast to a mild aggregation in MCAO animals. Therefore, it is suggested that MCAO should be a more suitable CP model than BCAO, based on the high survival rate, relatively-mild brain injury, and enough neurobehavioral disorders for the research on preventive and therapeutic compounds.
References
Altman, J., Sudarshan, K., Das, G.D., McCormick, N. and Barnes, D. (. 1971. ). The influence of nutrition on neural and behavioral development. 3. Development of some motor, particularly locomotor patterns during infancy. Dev. Psychobiol. 4:97–114.
Altman, J. and Sudarshan, K. (. 1975. ). Postnatal development of locomotion in the laboratory rat. Anim. Behav. 23:896–920.
Archer, L.N.J., Levene, M.I. and Evans, D.H. (. 1986. ). Cerebral artery doppler ultrasonography for prediction of outcome after perinatal asphyxia. Lancet. 2:1116–1118.
Back, S.A., Han, B.H., Luo, N.L., Chricton, C.A., Xanthoudakis, S., Tam, J., Arvin, K.L. and Holtzman, D.M. (. 2002. ). Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. 22:455–463.
Back, S.A., Luo, N.L., Borenstein, N.S., Levine, J.M., Volpe, J.J. and Kinney, H.C. (. 2001. ). Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 21:1302–1312.
Dawson ., D.A. ., Kusmoto K. and Graham, D.I. (1992) Inhibition nitric oxide synthesis does not reduce infarct volume in a rat model of focal cerebral ischemia. Neurosci. Lett. 142:151–154.
Delivoria-Papadopoulos, M. and Misbra, O.P. (. 1986. ). Mechanism of cerebral injury in perinatal asphyxia and strategies for prevention. J. Pediatr. 132:505–534.
Fan, L.W., Lin, S., Pang, Y., Lei, M., Zhang, F., Rhodes, P.G. and Cai, Z. (. 2005. ). Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav. Brain Res. 165:80–90.
Follett, P.L., Rosenberg, P.A., Volpe, J.J. and Jensen, F.E. (. 2000. ). NBQX attenuates excitotoxic injury in developing white matter. J. Neurosci. 20:9235–9241.
Hermans, R.H., Hunter, D.E., McGivern, R.F., Cain, C.D., and Longo, L.D. (. 1992. ). Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol. Teratol. 14:119–129.
Jesberger, J.A. and Richardson, J.S. (. 1991. ). Oxygen free radicals and brain dysfunction. Int. J. Neurosci. 57:1–17.
Kinney, H.C. and Back, S.A. (. 1998. ). Human oligodendroglial development, relationship to periventricular leukomalacia. Semin. Pediatr. Neurol. 5:180–189.
MacDonald, H.M., Mulligan, J.C. and Allen, A.C. (. 1980. ). Neonatal asphyxia: I. relationship to obstetric and neonatal complications to neonatal mortality in 38,405 consecutive deliveries. J. Pediatr. 96:898–902.
Michikawa, M., Lim, K. T., McLarnon, J. G. and Kim, S. U. (. 1994. ). Oxygen radical-induced neurotoxicity in spinal cord neuron cultures. J. Neurosci. Res. 37:62–70.
Mohr, J.P., Orgohozo, J.M., Harrison, M.J.G., Hennerici, M., Wahlgren, N.G. and Gelmers, J.H. (. 1994. ). Metaanalysis of oral nimodipine trials in acute ischemic stroke. Cerebrovasc. Dis. 4:197–203.
Mosmann, T. (. 1983. ). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J. Immunol. Methods. 65:55–63.
Mulligan, J.C., Painter, M.J. and O'Donoughue, P.A. (. 1980. ). Neonatal asphyxia: II. neonatal mortality and longterm sequelae. J. Pediatr. 96:903–907.
Palmer, C., Vannucci, R.C. and Towfigh, i. J. (. 1990. ). Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr. Res. 27:332–336.
Recker, R., Adami, A., Tone, B., Tian, H. R., Lalas, S., Hartman, R. E., Obenaus, A. and Ashwal, S. (. 2009. ). Rodent neonatal bilateral carotid artery occlusion with hypoxia mimics human hypoxic-ischemic injury. J. Cereb. Blood Folw Metab. 29:1305–1316.
Rice, J.E., Vannucci, R.C. and Brievley, J. B. (. 1981. ). The influence of immaturity on hypoxic-ischemic brain damage in the rats. Ann. Neurol. 9:131–141.
Rivkin, M.J., Flax, J., Mozell, R., Osathanondh, R., Volpe, J.J. and VillaKomaroff, L. (. 1995. ). Oligodendroglial development in human fetal cerebrum. Ann. Neurol. 38:92–101.
Roohey, T., Raju, T. and Mousetogiannis, A.N. (. 1997. ). Animal models for the study of perinatal hypoxicischemic encephalopathy, a critical analysis. Early Hum. Dev. 47:115–146.
Shen, Y., Isaacson, R.L. and Smotherman, W.P. (. 1991. ). The behavioral and anatomical effects of prenatal umbilical cord clamping in the rat and their alteration by the prior maternal administration of nimodipine. Restor. Neurol. Neurosci. 3:11–22.
Towfighi, J., Yager, J.Y., Housman, C. and Vannucci, R.C. (. 1991. ). Neuropathology of remote hypoxic-ischemic damage in the immature rat. Acta Neuropathol. 81:578–587.
Warner, B.B., Stuart, L.A., Papes. R.A. and Wispé, J.R. (. 1998. ). Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am. J. Physiol. 275:110–117.
Figure 1.
Righting reflex of normal (●), monolateral carotid artery occlusion (MCAO, ▼) and bilateral carotid artery occlusion (BCAO, ■) rats. Rats were subjected to hypoxia-ischemia operation on postnatal day 4 (PND4), and to righting reflex performances on PND8–21. A, male; B, female.
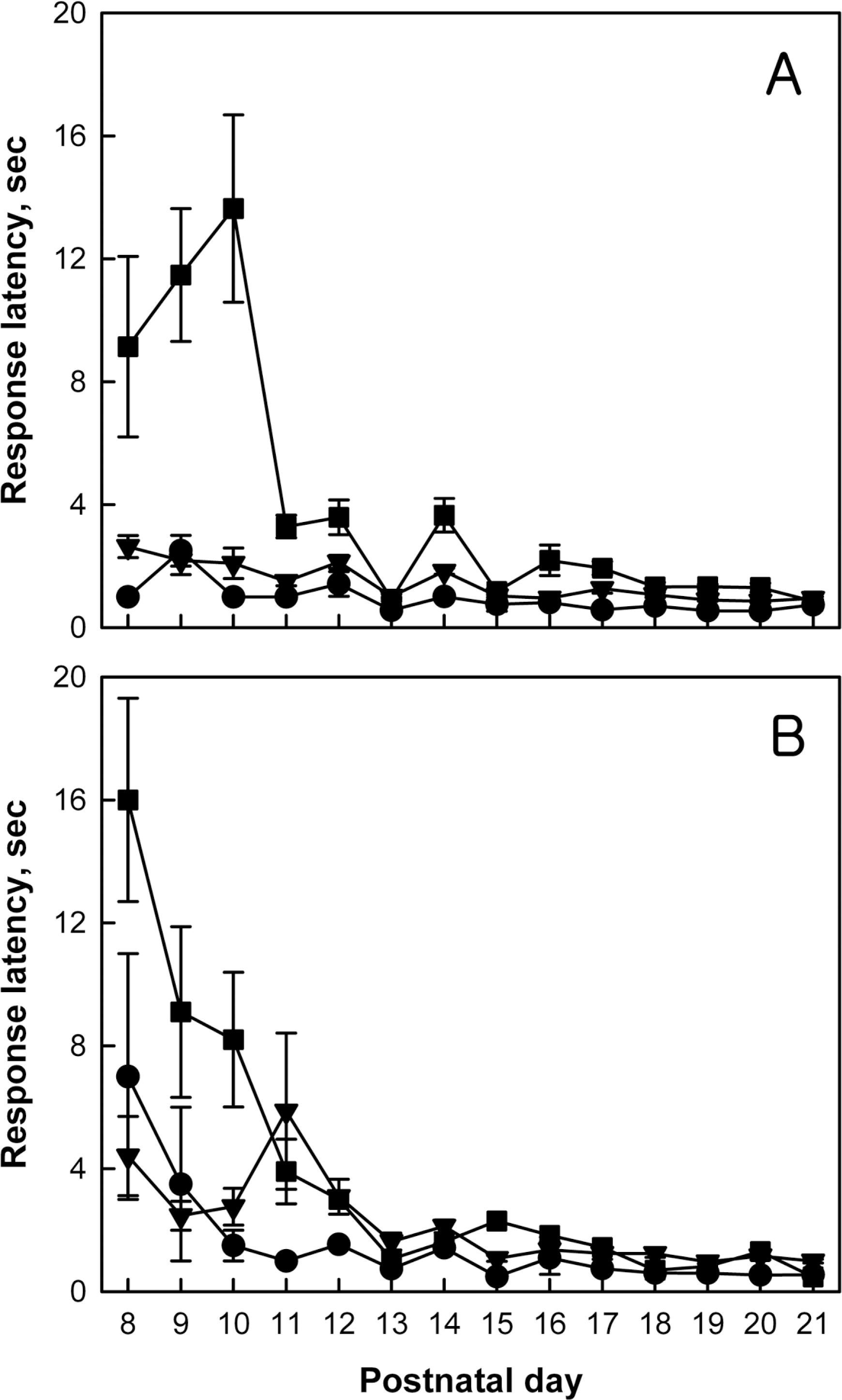
Figure 2.
Cliff avoidance reflex of normal (●), monolateral carotid artery occlusion (MCAO, ▼) and bilateral carotid artery occlusion (BCAO, ■) rats. Rats were subjected to hypoxia-ischemia operation on postnatal day 4 (PND4), and to cliff avoidance performances on PND8–21. Cut-off time was set at 60 sec. A, male; B, female.
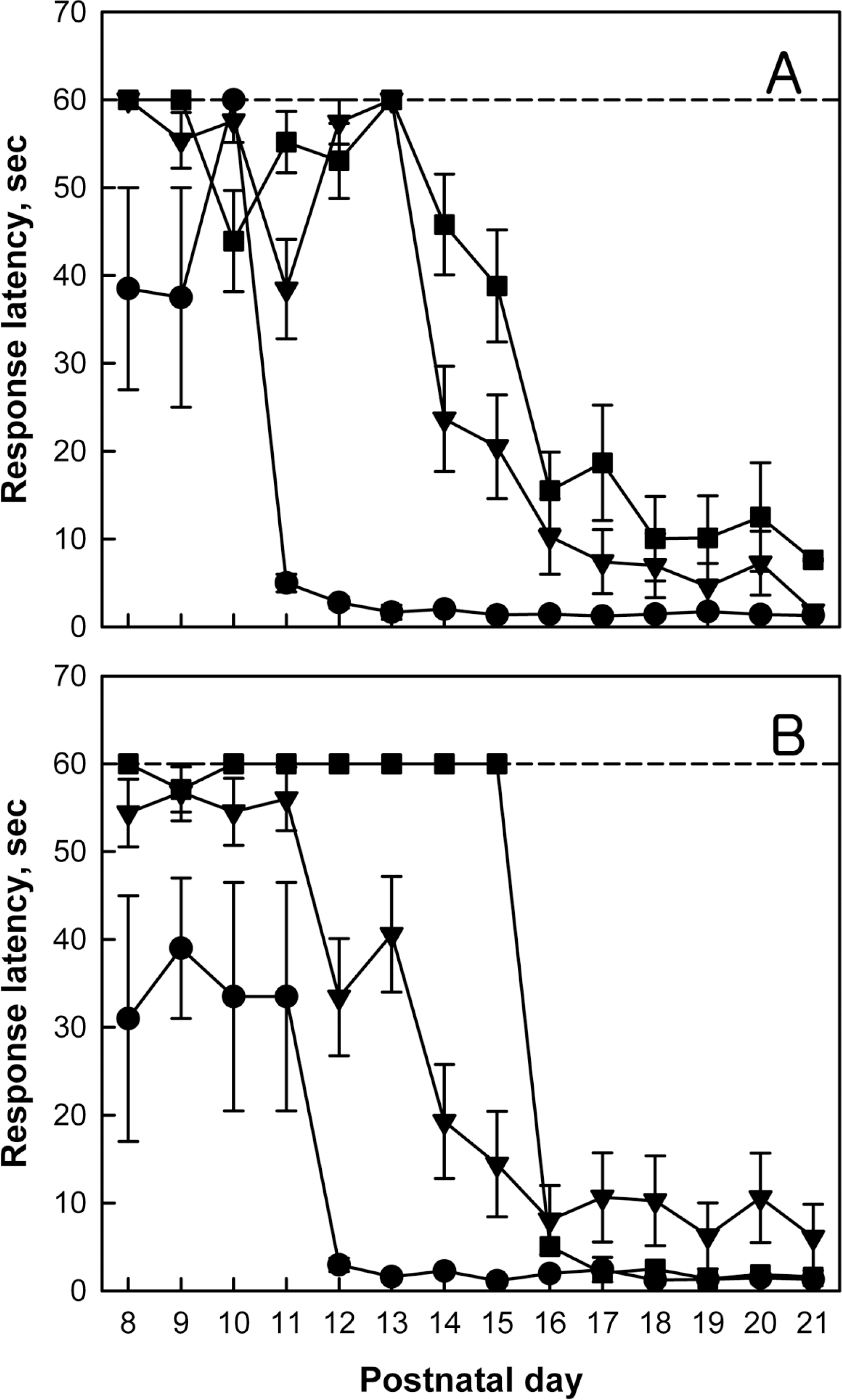
Figure 3.
Locomotor activity of normal (●), monolateral carotid artery occlusion (MCAO, ▼) and bilateral carotid artery occlusion (BCAO, ■) rats. Rats were subjected to hypoxia-ischemia operation on postnatal day 4 (PND4), and locomotor activity was monitored for 5 min on PND9–12.
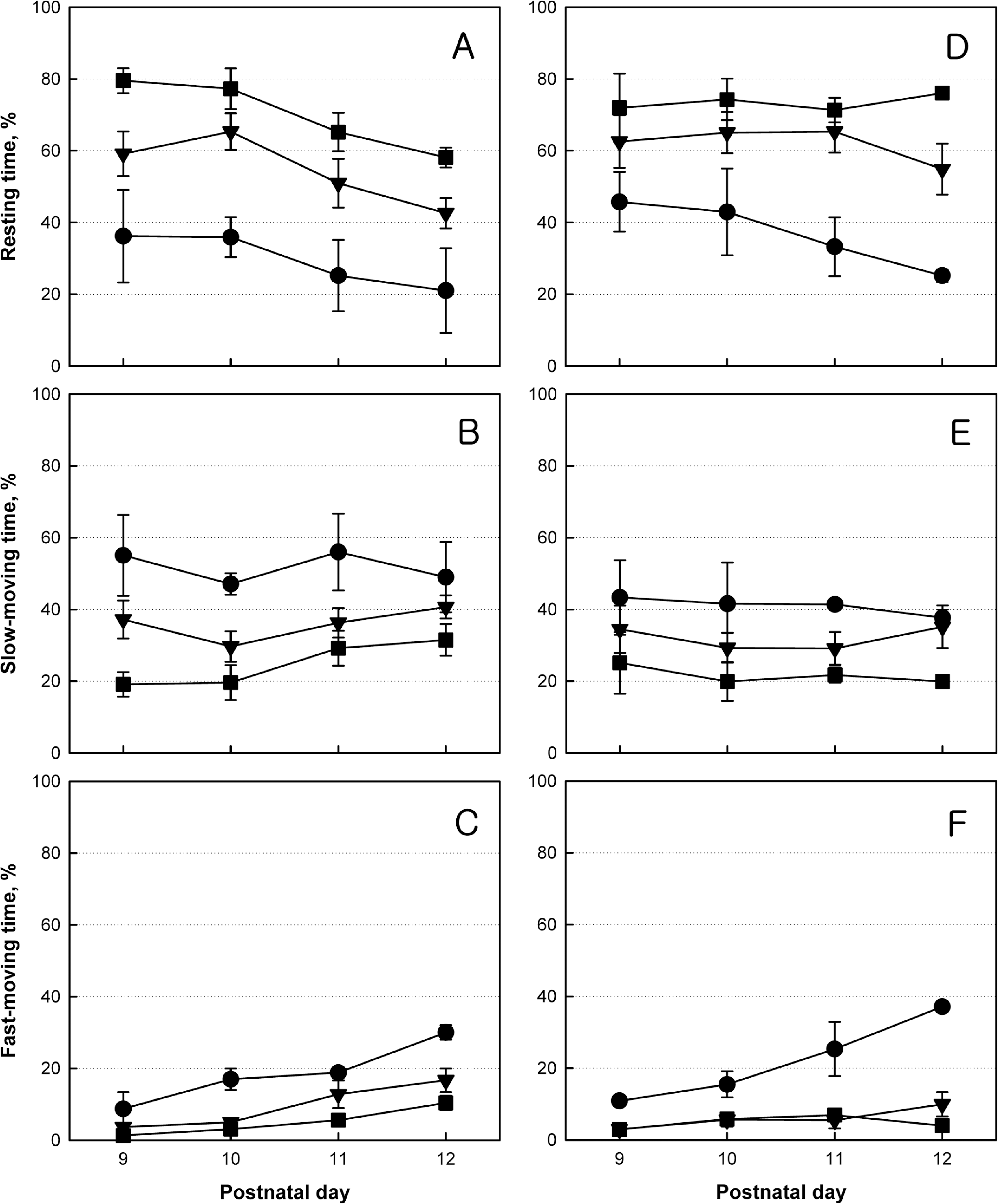
Figure 4.
Learning and memory function of normal (●), monolateral carotid artery occlusion (MCAO, ▼) and bilateral carotid artery occlusion (BCAO, ■) rats. Rats were subjected to hypoxia-ischemia operation on postnatal day 4 (PND4), and to passive avoidance performances on PND18–21. Cut-off time was set at 300 sec. A, male; B, female.
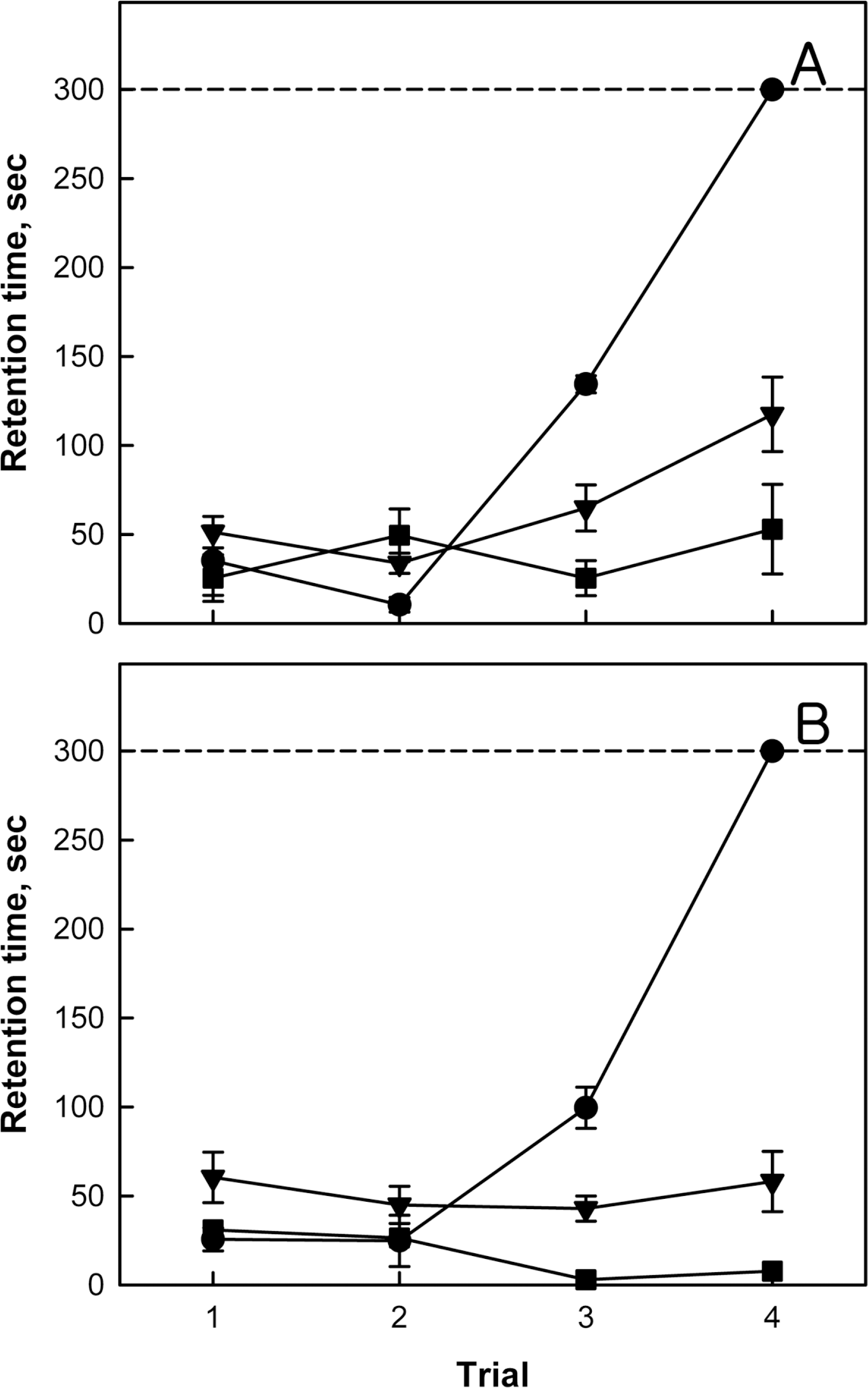
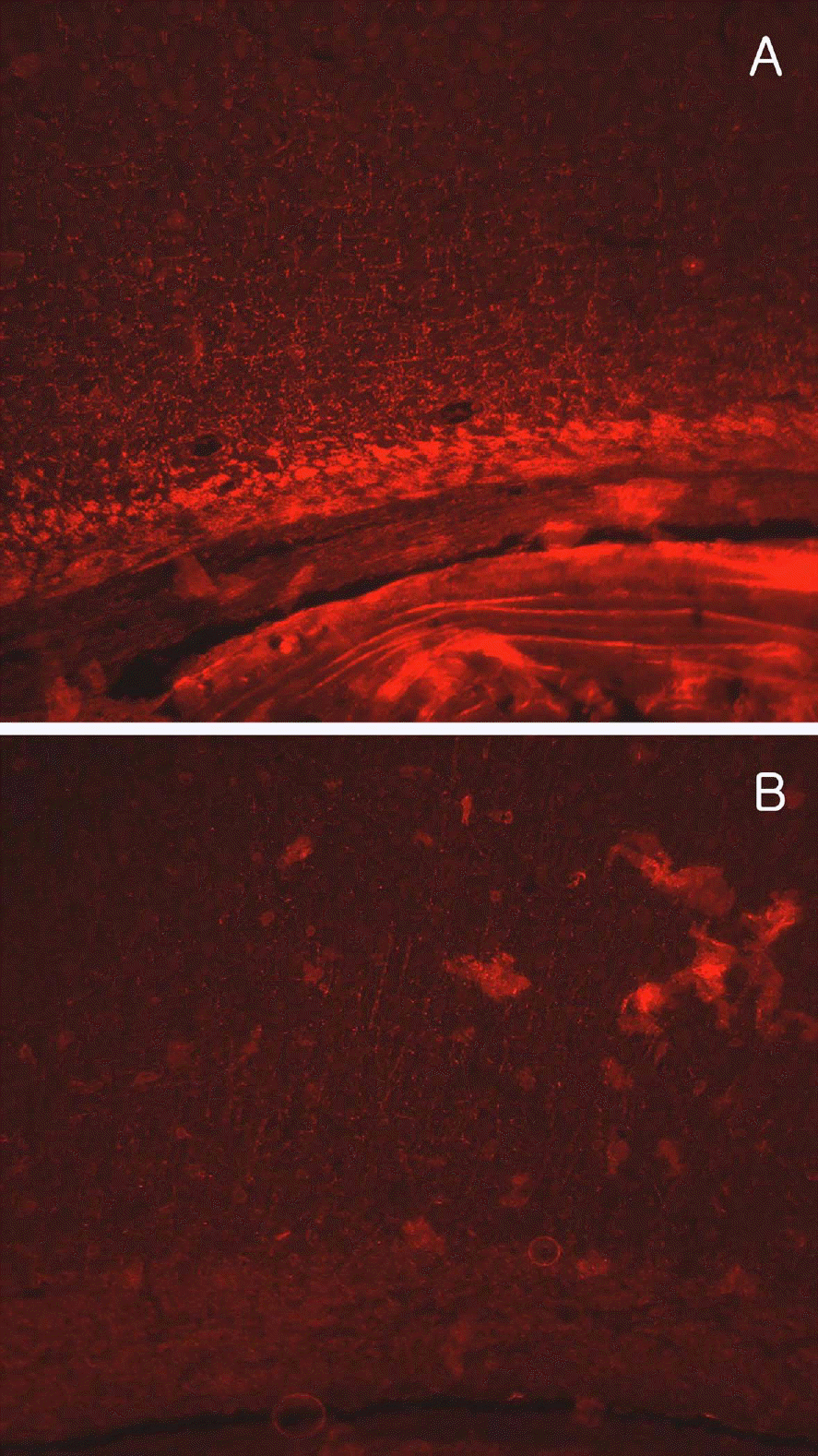
Figure 5.
Representative photomicrographs of myelin basic protein (MBP) immunostaining in the periventricular regions of monolateral carotid artery occlusion (MCAO, A) and bilateral carotid artery occlusion (BCAO, B) rats. Note the red fluorescence of MBP, showing mild aggregation in MCAO and severe degradation and loss in BCAO rats.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download