Abstract
The leaner mouse carries a mutation in the gene encoding the α1A subunit of P/Q-type calcium channels. Leaner mice exhibit extensive cerebellar granule and Purkinje cell loss that results in cerebellar dysfunction. A previous study suggested that a small population of leaner Purkinje cells undergo apoptosis, however the cell death mode of the rest of degenerating Purkinje cells has not been identified. In order to investigate the mechanisms underlying leaner Purkinje cell death, gene arrays that contain 243 cell death related genes were carried out. To increase the chance of detecting Purkinje cell specific genes, laser capture microdissection was employed to obtain Purkinje cell enriched samples. The gene array analysis revealed several potential genes that are involved in autophagic cell death pathway including cathepsin D, a key lysosomal protease that triggers autophagic degradation. Further analysis on LC3, which is a hallmark for autophagic cell death showed that leaner Purkinje cells are degenerating via autophagic process. The present study provides evidence that calcium channel defects trigger different modes of neurodegeneration in the cerebellum.
REFERENCES
Burgess D.L.., Noebels J.L.1999. ).Single gene defects in mice: the role of voltage-dependent calcium channels in absence models. Epilepsy Res. 36(2-3):111–122.

Chakrabarti L.., Eng J.., Ivanov N.., Garden G.A.., La Spada A.R.2009. Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Mol. Brain. 2(1):24.

Dove L.S.., Abbott L.C.., Griffith W.H.1998. Whole-cell and single-channel analysis of P-type calcium currents in cerebellar Purkinje cells of leaner mutant mice. J. Neurosci. 18(19):7687–7699.

Dove L.S.., Nahm S.S.., Murchison D.., Abbott L.C.., Griffith W.H.2000. Altered calcium homeostasis in cerebellar Purkinje cells of leaner mutant mice. J. Neurophysiol. 84(1):513–524.

Fletcher C.F.., Lutz C.M.., O'Sullivan T.N.., Shaughnessy J.D. Jr.., Hawkes R.., Frankel W.N.., Copeland N.G.., Jenkins N.A.1996. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 87(4):607–617.

Frank-Cannon T.C.2005. Texas A&M University Dissertation, pp. 74-100, Texas A&M University, College Station.
Herrup K.., Wilczynski S.L.1982. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience. 7(9):2185–2196.

Hong N.A.., Flannery M.., Hsieh S.N.., Cado D.., Pedersen R.., Winoto A.2000. Mice lacking Dad1, the defender against apoptotic death-1, express abnormal N-linked glycoproteins and undergo increased embryonic apoptosis. Dev. Biol. 220(1):76–84.

Ito M.1984. The Cerebellum and Neural Control, pp. 21–39. Raven Press, New York.
Kerr M.K.., Martin M.., Churchill G.A.2000. Analysis of variance for gene expression microarray data. J. Comput. Biol. 7(6):819–837.

Klionsky D.J.2007. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8(11):931–937.

Krajewska M.., Mai J.K.., Zapata J.M.., Ashwell K.W.., Schendel S.L.., Reed J.C.., Krajewski S.2002. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax and Bak during development of murine nervous system. Cell Death Differ. 9(2):145–157.

Lau F.C.., Frank T.C.., Nahm S.S.., Stoica G.., Abbott L.C.2004. Postnatal apoptosis in cerebellar granule cells of homozygous leaner (tg1a/tg1a) mice. Neurotox. Res. 6(4):267–280.
Levine B.., Yuan J.2005. Autophagy in cell death: an innocent convict? J. Clin. Invest. 115(10):2679–2688.

Mori Y.., Wakamori M.., Oda S.., Fletcher C.F.., Sekiguchi N.., Mori E.., Copeland N.G.., Jenkins N.A.., Matsushita K.., Matsuyama Z.., Imoto K.2000. Reduced voltage sensitivity of activation of P/Q-type Ca2+ channels is associated with the ataxic mouse mutation rolling Nagoya (tg(rol)). J. Neurosci. 20(15):5654–5662.
Mullen R.J.., Eicher E.M.., Sidman R.L.1976. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc. Natl. Acad. Sci. USA. 73(1):208–212.

Nahm S.S.., Tomlinson D.J.., Abbott L.C.2002. Decreased calretinin expression in cerebellar granule cells in the leaner mouse. J. Neurobiol. 51(4):313–322.

Nishiyama J.., Matsuda K.., Kakegawa W.., Yamada N.., Motohashi J.., Mizushima N.., Yuzaki M.2010. Reevaluation of neurodegeneration in lurcher mice: constitutive ion fluxes cause cell death with, not by, autophagy. J. Neurosci. 30(6):2177–2187.

Ophoff R.A.., Terwindt G.M.., Frants R.R.., Ferrari M.D.1998. P/Q-type Ca2+ channel defects in migraine, ataxia and epilepsy. Trends Pharmacol. Sci. 19(4):121–127.

Selimi F.., Lohof A.M.., Heitz S.., Lalouette A.., Jarvis C.I.., Bailly Y.., Mariani J.2003. Lurcher GRID2-induced death and depolarization can be dissociated in cerebellar Purkinje cells. Neuron. 37(5):813–819.

Vicencio J.M.., Lavandero S.., Szabadkai G.2010. Ca2+, autophagy and protein degradation: thrown off balance in neurodegenerative disease. Cell Calcium. 47(2):112–121.

Zuo J.., De Jager P.L.., Takahashi K.A.., Jiang W.., Linden D.J.., Heintz N.1997. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 388(6644):769–773.
Figure 1.
Photomicrographs show dissections of cerebellar Purkinje cells (white arrows) using laser capture microdissection. Pictures show before (A) and after (B) laser capture microdissection on an unstained cerebellar section and procured Purkinje cells on the transfer film (C). GCL, granule cell layer; WM, white matter.
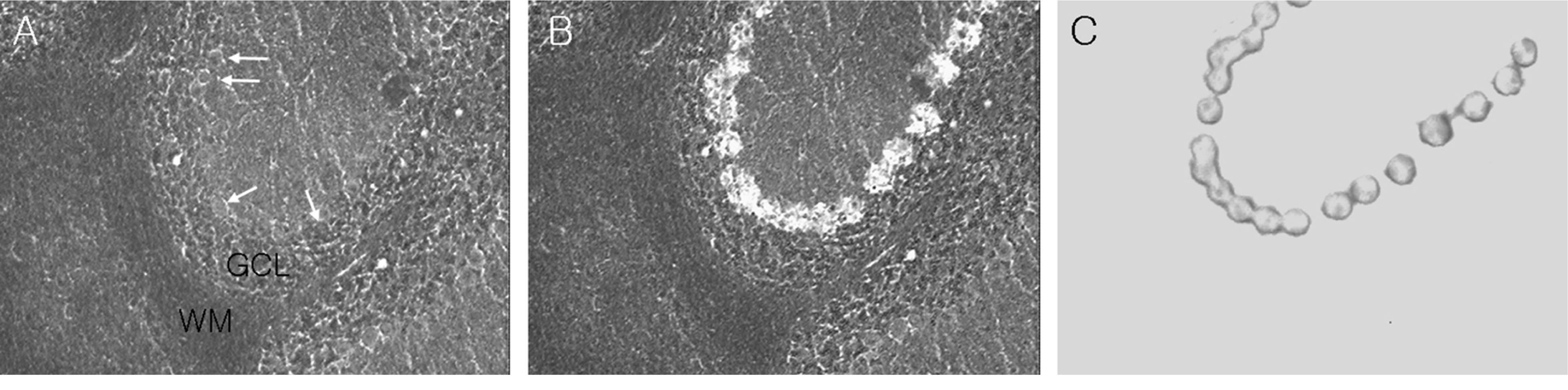
Figure 2.
Western blot analysis of cathepsin D expression in wild type and leaner mice (A). Note that higher cathepsin D expression near 30 kDa in the leaner cerebellum. Densitometric analysis of cathepsin D normalized by b-actin. Leaner mice showed a significant increase in cathepsin D expression (B). Data are presented as mean±SEM. ∗P<0.05.
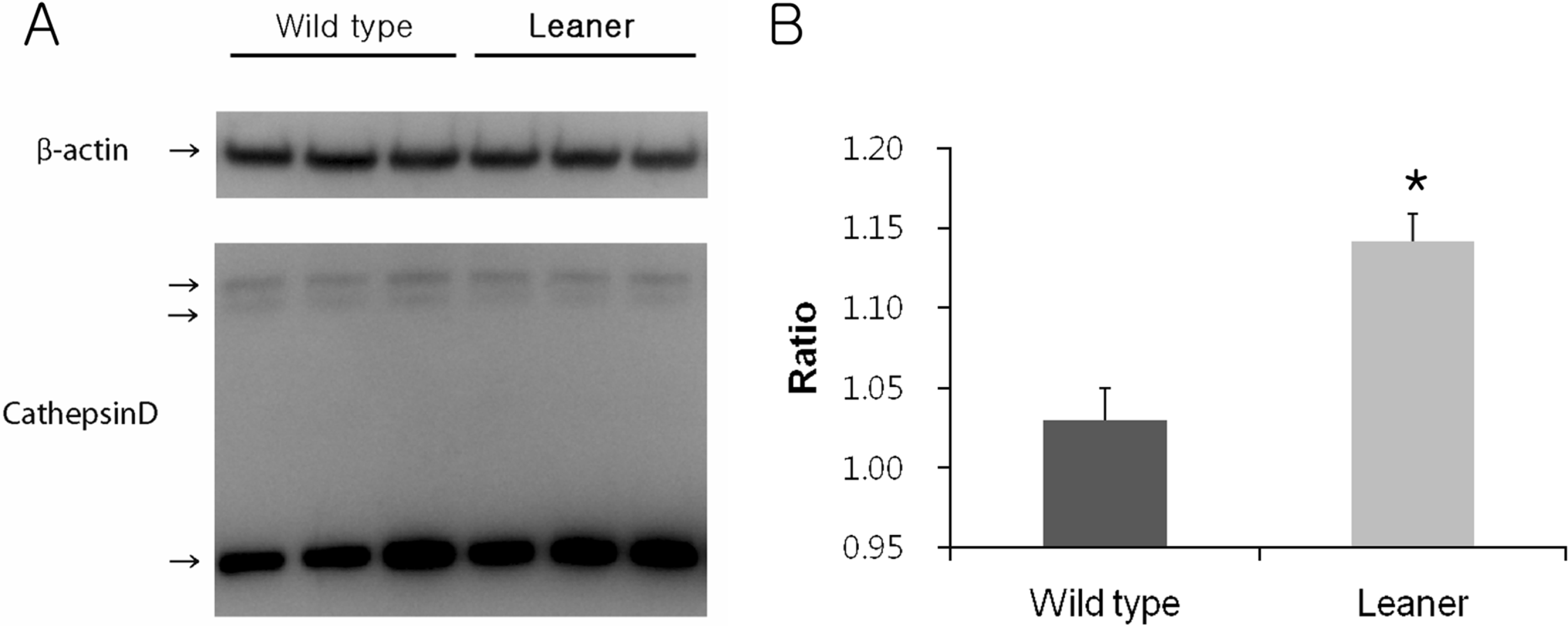
Figure 3.
Photomicrographs of cathepsin D immunohistochemistry of wild type (A) and leaner mouse (B). Note that the leaner cerebellum shows increased cathepsin D immunoreactivity in the Purkinje cell bodies. ML, molecular layer; PCL, Purkinje cell layer; GCL, granule cell layer. Scale bar=20 µm.
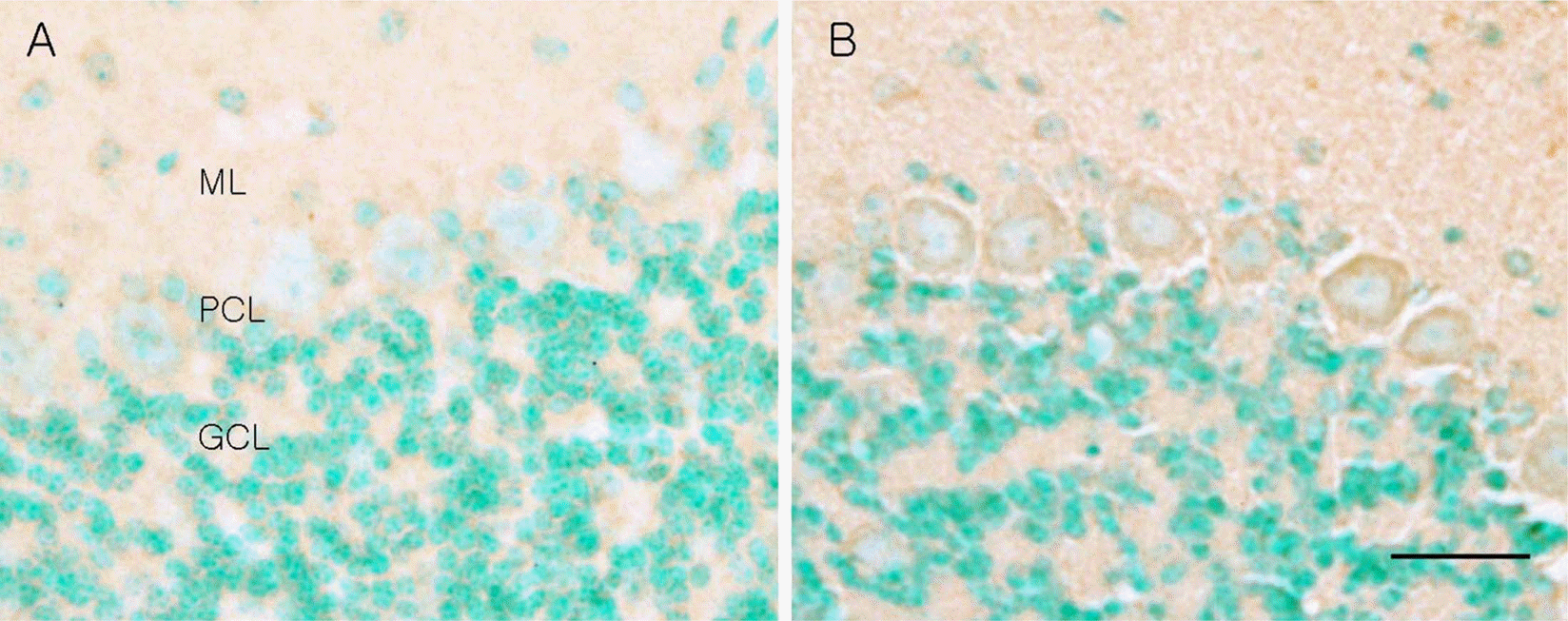
Figure 4.
Photomicrographs of LC3 immunohistochemistry of wild type (A) and leaner mouse (B). Note that the leaner cerebellum shows increased LC3 immunoreactivity in the Purkinje cell bodies. ML, molecular layer; PCL, Purkinje cell layer; GCL, granule cell layer. Scale bar=20 µm.
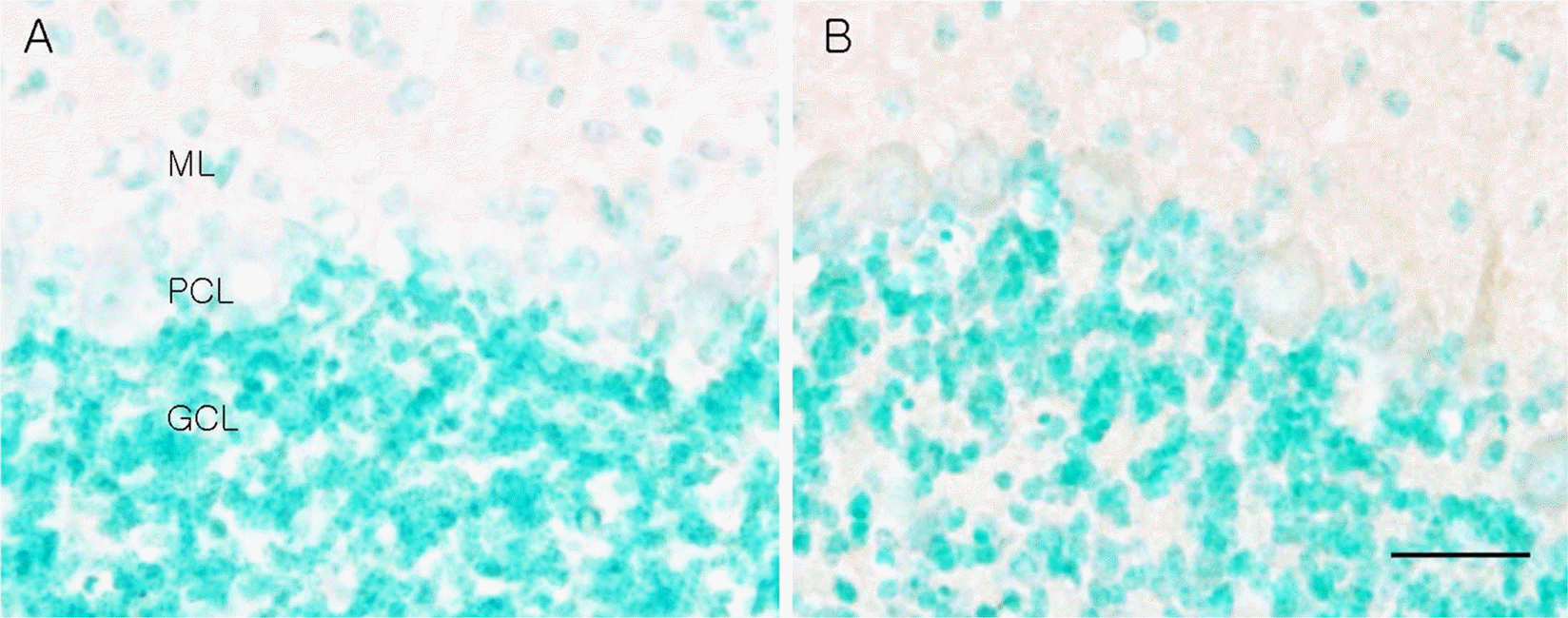
Table 1.
Up-regulated genes in leaner Purkinje cells∗




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download