Abstract
The protective efficacy of a silkworm extract (SE) on N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism and its possible mechanisms were studied in C57BL/6 mice. Mice were administrated intraperitoneally with SE (20 mg/kg/day) for 15 days and MPTP (10 mg/kg/day) was administrated subcutaneously into the mice for the first 6 consecutive days 1 hour before SE treatment. All animals were sacrificed 24 hours after the last SE treatment. Then the parameters related to general toxicity and neurobiochemical markers, such as the dopamine level and the activities of monoamine oxidase (MAO)-B, were measured in various regions of the brain. Treatment of mice with SE effectively attenuated the MPTP-induced decline of striatal dopamine level. MAO-B activity in SE-pretreated mice was inhibited in whole brain, cerebellum and substantia nigra. These results suggest that SE plays an effective role in attenuating MPTP-induced neurotoxicity in animal model. These neuroprotective effects of SE are likely the result from the inhibitory effect on MAO-B activity in mouse brain.
Go to : 
REFERENCES
Alexi T.., Borlongan C.V.., Faull R.L.M.., Williams C.E.., Clark R.G.., Gluckman P.D.., Hughes P.E.2000. Neuroprotective strategies for basal ganglia degeneration: Parkinson's and Huntington's diseases. Prog. Neurobiol. 60(5):409–470.

Chiba K.., Trevor A.., Castagnoli N.J.1984. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Biophys. Res. Commun. 120(2):574–578.

Chung S.H.., Ryu J.H.., Kim I.J.1996. Blood glucose lowering of silkworm. J. Hyunghee Pharm. 24:95–100.
Ferger B.., Teismann P.., Earl C.D.., Kuschinsky K.., Oertel W.H.2000. The protective effects of PBN against MPTP toxicity are independent of hydroxyl radical trapping. Phamacol. Biochem. Behav. 65(3):425–431.

Foley P.., Gerlach M.., Youdim M.B.., Rieder P.2000. MAO-B inhibitors: multiple roles in the therapy of neurodegenerative disorders. Parkinsonism Relat. Disord. 6(1):25–47.

Kalaria R.N.., Mitchell M.J.., Harik S.I.1987. Correlation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity with blood brain barrier monoamine oxidase activity. Proc. Natl. Acad. Sci. USA. 84(10):3521–3525.
Kang X.., Chen J.., Xu Z.., Li H.., Wang B.2007. Protective effects of Ginkgo biloba extract on paraquat-induced apoptosis of PC12 cells. Toxicol. In Vitro. 21(6):1003–1009.

Kang Y.K.., Choo S.K.., Nam S.H.2001. Effects of silkworm-extract on blood components levels in mice. Korean J. Entomol. 31(4):243–247.
Kang Y.K.., Lim H.B.., Sohn H.O.., Lee Y.G.., Lee D.W.., Nam S.H.2000. Effect of silkworm-extract on mucus secretion in rat tracheobronchial lumen. Korean J. Entomol. 30(2):71–75.
Kang Y.K.., Nam S.H.., Sohn H.O.., Lee D.W.2005. Inhibitory effects of silkworm-extract (SE) on monoamine oxidase activity in vitro and in vivo. Entomol. Res. 35(3):189–193.
Kasture S.., Pontis S.., Pinna A.., Schintu N.., Spina L.., Longoni R.., Simola N.., Ballero M.., Morelli M.2009. Assessment of symptomatic and neuroprotective efficacy of Mucuna pruriens seed extract in rodent model of Parkinson's disease. Neurotox. Res. 15(2):111–122.

Kim M.S.., Choue R.W.., Chung S.H.., Koo S.J.1998. Blood glucose lowering effects mulberry leaves and silkworm extracts an mice fed with high-carbohydrate diet. Korean Nutr. Soc. 31(2):117–125.
Koutsilieri E.., Chen T.S.., Rausch W.D.., Riederer P.1996. Selegiline is neuroprotective in primary brain cultures treated with 1-methyl-4-phenylpyridinium. Eur. J. Pharmacol. 306(1-3). 181–186.
Langstone J.W.., Ballard P.., Tetrud J.W.., Irwin I.1983. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 219(4587):979–980.

Levites Y.., Weinreb O.., Maor G.., Youdim M.B.., Mandel S.2001. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6- terahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 78(5):1073–1082.
Li Q.Y.., Pedersen C.., Day B.J.., Petal M.2001. Dependence of excitotoxic neurodegeneration on mitochondrial aconitase inactivation. J. Neurochem. 78(4):746–755l.

Lowry O.H.., Rosebrough H.J.., Farr A.L.., Randall R.J.1951. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1):265–275.
Mohanasundari M.., Srinivasan M.S.., Sethupathy S.., Sabesan M.2006. Enhanced neuroprotective effect by combination of bromocriptine and Hypericum perforatum extract against MPTP-induced neurotoxicity in mice. J. Neurol Sci. 249(2):140–144.

Mohanakumar K.P.., Muralikrishnan D.., Thomas B.2000. Neuroprotection by sodium salicylate against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Brain Res. 864(2):281–290.

Nicklas W.J.., Vyas I.., Heikkila R.E.1985. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenylpyridinium, a metabolite of the neurotoxine, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 36(26):2503–2508.
Rojas P.., Serrano-Garca N.., Mares-Smano JJ.., Medina-Campos O.N.., Pedraza-Chaverri J.., Ogren S.O.2008. EGb761 protects against nigrostriatal dopaminergic neurotoxicity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice: role of oxidative stress. Eur. J. Neurosci. 28(1):41–50.

Ryu K.S.., Lee H.S.., Chung S.H.., Kang P.D.1997. An activity of lowering blood-glucose levels according to preparative conditions of silkworm power. Korean. J. Seric. Sci. 39(1):79–85.
Van Kampen J.., Robertson H.., Hagg T.., Drobitch R.2003. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson's disease. Exp. Neurol. 184(1):521–529.

Widdowson P.S.., Farnworth M.J.., Upton R.., Simpson M.G.1996. No changes in behavior, nigrostriatal system neurochemistry or neuronal cell death following toxic multiple oral paraquat administration to rats. Hum. Exp. Toxicol. 15(7):583–591.
Wu W.R.., Zhu X.Z.1999. Involvement of monoamine oxidase inhibition in neuroprotective and neurorestorative effects of Ginkgo biloba extract against MPTP-induced nigrostriatal dopaminergic toxicity in C57 mice. Life Sci. 65(2):157–164.

Zou L.., Xu J.., Jankovic J.., He Y.., Appel S.H.., Le W.2000. ).Pramipexole inhibitits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neurosci. Lett. 281(2-3):167–170.
Go to : 
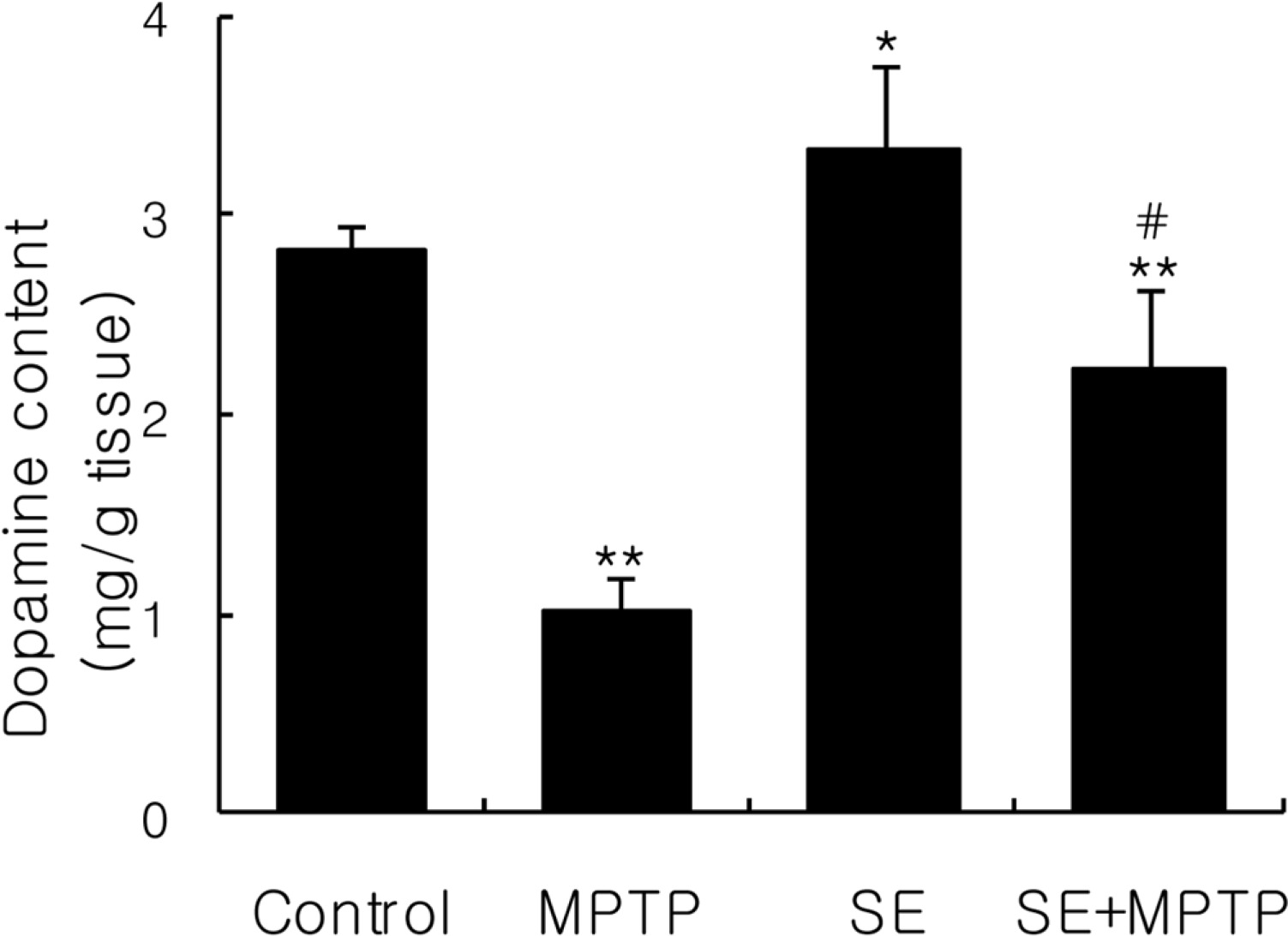 | Figure 1.Effect of silkworm extract (SE) pretreatment against depletion of striatal dopamine levels by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Striatal dopamine level was determined by high performance lipid chromatography and expressed as µg/g wet striatal tissue. Data were expressed mean±SD of 10 animals per group. ∗,∗∗Significantly different from the control group at P<0.05 and P<0.01, respectively. #Significantly different from the MPTP-treated group at P<0.01. |
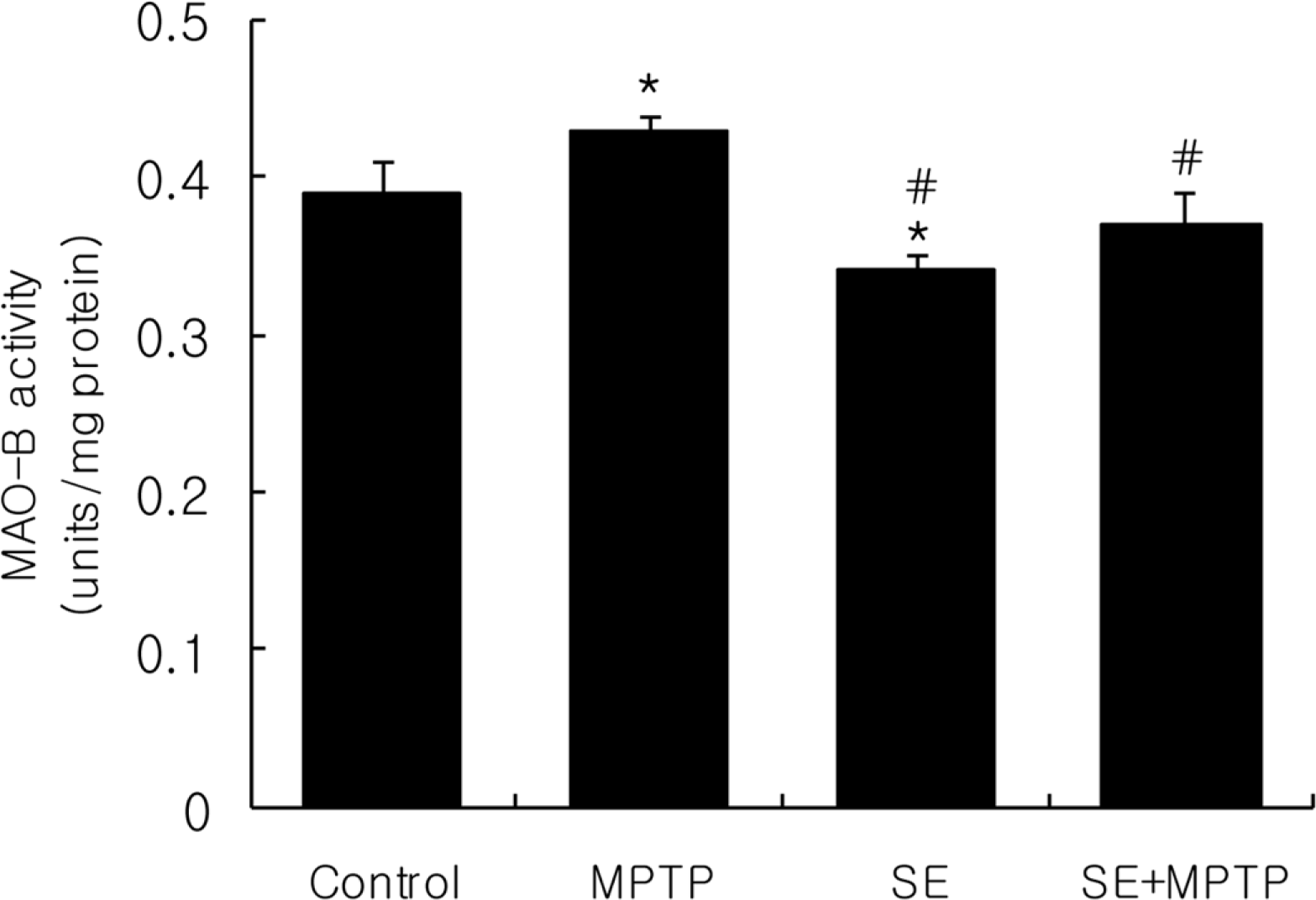 | Figure 2.Effect of silkworm extract (SE) or/and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment on monoamine oxidase-B activity in the whole brain of mice. Data were expressed as mean±SD of six repeated experiments using whole brain mitochondrial fractions pooled from 8 mice. ∗Significantly different from the control group at P<0.05. #Significantly different from MPTP treated group at P<0.05. |
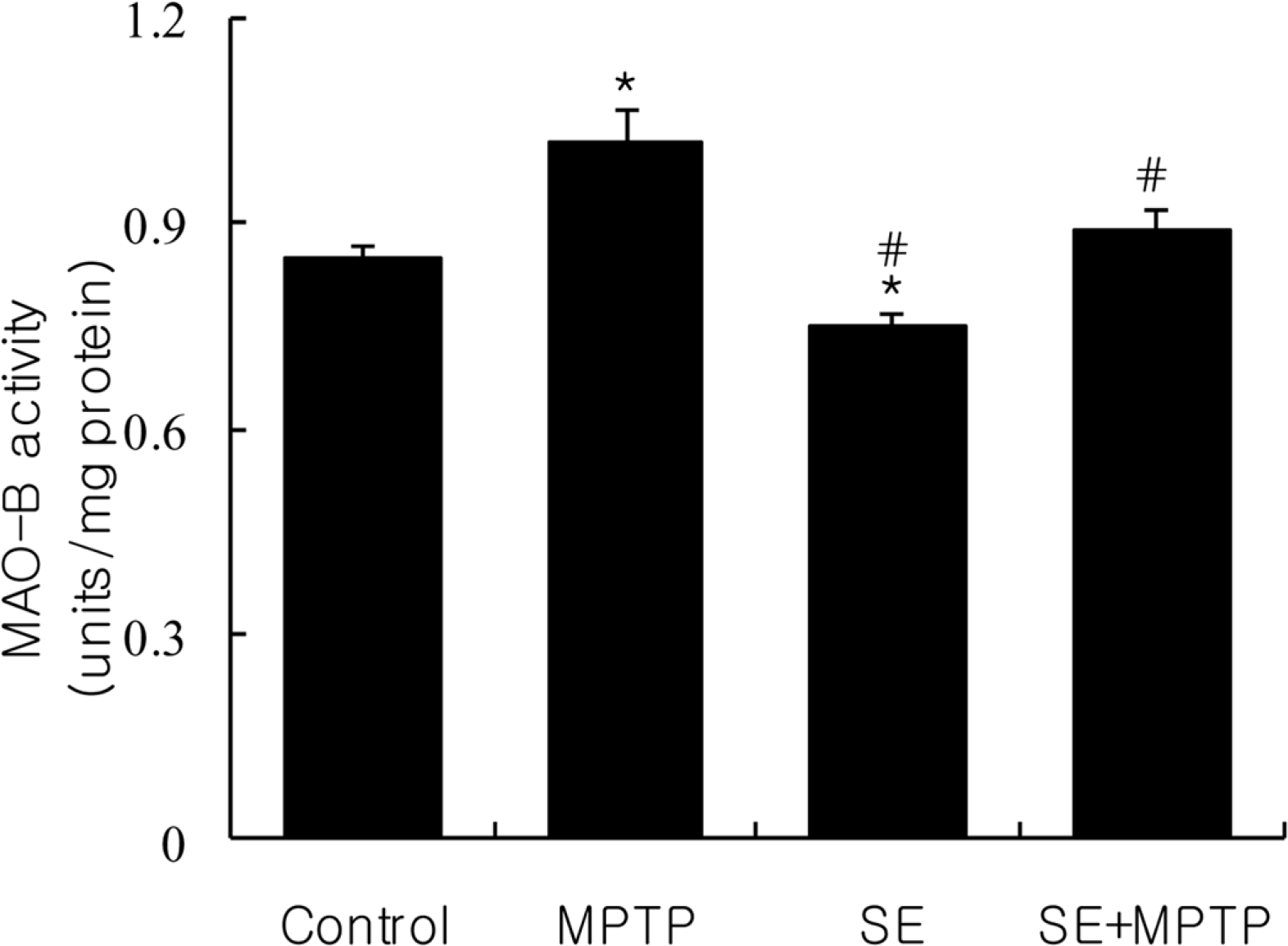 | Figure 3.Effect of silkworm extract (SE) or/and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment on monoamine oxidase-B activity in the cerebellum of mice. Data were expressed as mean±SD of six repeated experiments using cerebellum mitochondrial fractions pooled from 8 mice. ∗Significantly different from the control group at P<0.01. #Significantly different from MPTP-treated group at P<0.01. |
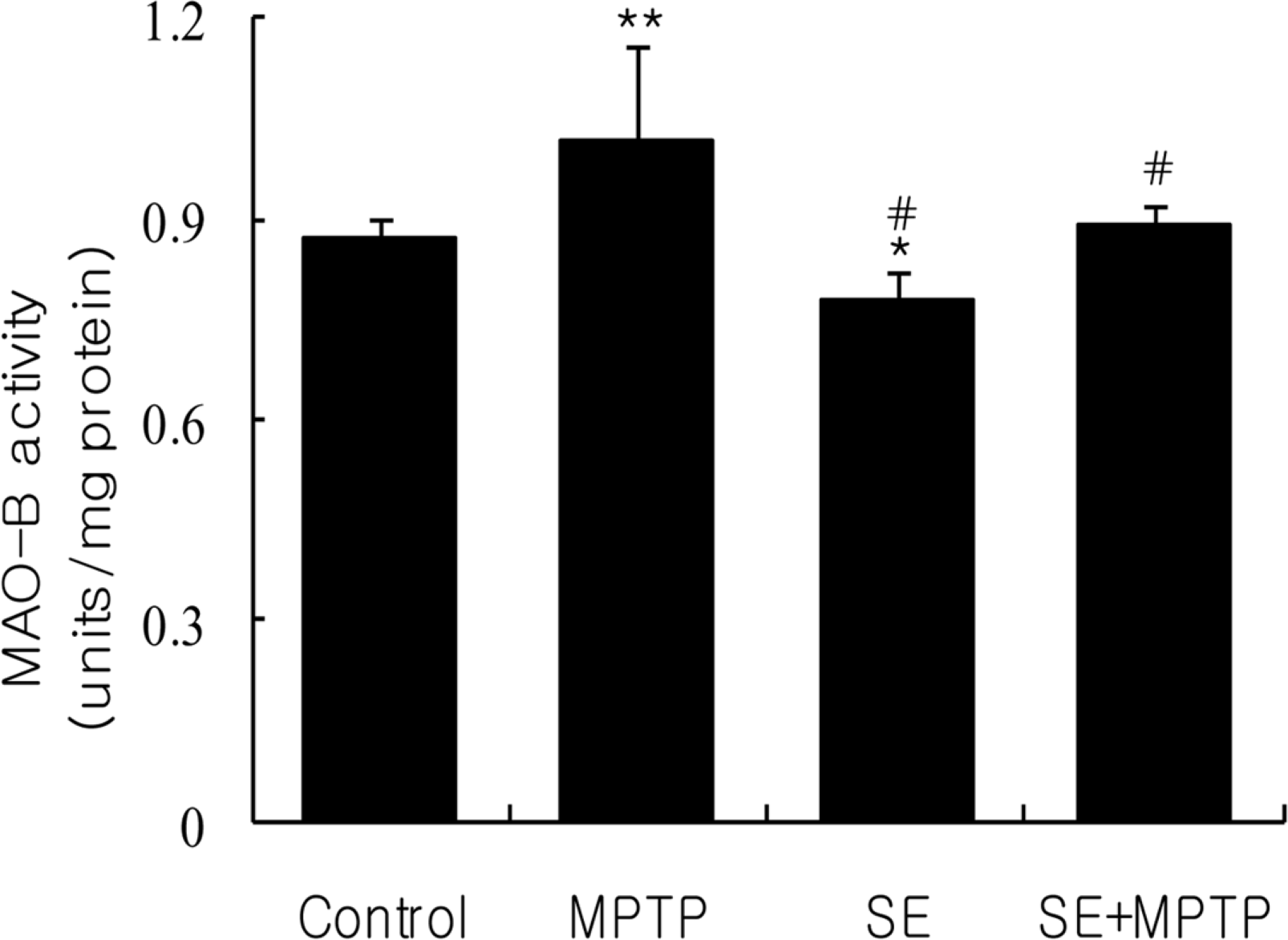 | Figure 4.Effect of silkworm extract (SE) or/and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment on monoamine oxidase-B activity in the substantia nigra of mice. Data were expressed as mean±SD of six repeated experiments using substantia nigra mitochondrial fractions pooled from 8 mice. ∗,∗∗Significantly different from the control group at P<0.05 and P<0.01, respectively. #Significantly different from MPTP-treated group at P<0.01. |
Table 1.
Effect of silkworm extract (SE) treatment on relative organs weights to body weight of mice
| Treatment | Weights (g) | |||
|---|---|---|---|---|
| Body | Brain | Liver | ||
| Day 1 | Day 16 | |||
| Vehicle | 22.3±0.8 | 23.2±0.9 | 0.48±0.02 | 0.93±0.10 |
| SE (20 mg/kg) | 24.6±2.4 | 24.1±0.8 | 0.47±0.02 | 0.87±0.07 |
Table 2.
Effect of silkworm extract (SE) treatment on glutamic oxalacetic transaminase (GOT) and glutamic pyruvate transaminase (GPT) activities in the serum of mice
| Treatment | GOT (IU/L) | GPT (IU/L) |
|---|---|---|
| Vehicle | 114±14 | 42±5.7 |
| SE (20 mg/kg) | 121±23 | 39±5.8 |
Table 3.
Condition of high performance lipid chromatography




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download