Abstract
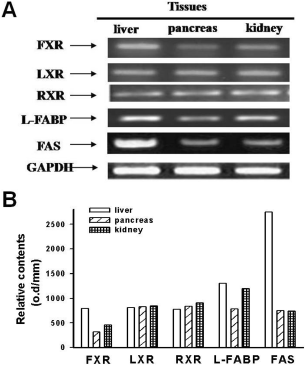
The miniature pig is a very suitable donor species in xenotransplantation of human organs. Lipid metabolism is an important process that involves the creation and degradation of lipids, which is associated with the function of the gastro-intestinal tract. However, the distribution of lipid metabolism related molecules in the gastro-intestinal tract in the miniature pig is unclear. The present study examined the expression of farnesoid X-receptor (FXR), liver X- receptor (LXR), retinoid X-receptor (RXR), liver fatty acid binding protein (L-FABP), fatty acid synthase (FAS) mRNA in the digestive organs of miniature pigs. FXR and LXR mRNA were not expressed in the stomach but were expressed at high and low density in the small and large intestines, respectively. RXR mRNA was expressed in stomach with moderate density, small intestine with high density and in the large intestine with low density. L-FABP and FAS mRNA were expressed in the stomach and large intestine with low density and in the small intestine with high density. L-FABP mRNA was expressed in the liver and kidney with high density, and in pancreas with low density. FAS mRNA was expressed in the liver with high density, and in pancreas and kidney with low density.
Go to : 
REFERENCES
Canbay A.., Bechmann L.., Gerken G.2007. Lipid metabolism in the liver. J. Gastroenterol. 45(1):35–41.

Cantafora A.., Blotta I.., Rivabene R.., Pisciotta L.., Bertolini S.2003. Evaluation of RNA messengers involved in lipid trafficking of human intestinal cells by reverse-transcription polymerase chain reaction with competimer technology and microchip electrophoresis. Electrophoresis. 24(21):3748–3754.

Clarke S.D.1993. Regulation of fatty acid synthase gene expression: an approach for reducing fat accumulation. J. Anim Sci. 71(7):1957–1965.

Desvergne B.2007. RXR: from partnership to leadership in metabolic regulations. Vitam. Horm. 75:1–32.

Feig J.E.., Quick J.S.., Fisher E. A.2009. ).The role of a murine transplantation model of atherosclerosis regression in drug discovery. Curr. Opin. Invest. Drugs. 10(3):232–238.
Forman B.M.., Goode E.., Chen J.., Oro A.E.., Bradley D.J.., Perlmann T.., Noonan D.J.., Burka L.T.., McMorris T.., Lamph W.W.., Evans R.M.., Weinberger C.1995. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 81:687–693.

Heimerl S.., Moehle C.., Zahn A.., Boettcher A.., Stremmel W.., Langmann T.., Schmitz G.2006. Alterations in intestinal fatty acid metabolism in inflammatory bowel disease. Biochim. Biophys. Acta 1762. (3):341–350.
Higashiyama H.., Kinoshita M.., Asano S.2008. Immuno-localization of farnesoid X receptor (FXR) in mouse tissues using tissue microarray. Acta Histochem. 110:86–93.

Hulzebos C.V.., Bijleveld C.M.., Stellaard F.., Kuipers F.., Fidler V.., Slooff M.J.., Peeters P.M.., Sauer P.J.., Verkade H.J.2004. ).Cyclosporine A-induced reduction of bile salt synthesis associated with increased plasma lipids in children after liver transplantation. Liver Transpl. 10(7):872–880.

Inoue J.., Satoh S.., Kita M.., Nakahara M.., Hachimura S.., Miyata M.., Nishimaki-Mogami T.., Sato R.2008. PPAR alpha gene expression is up-regulated by LXR and PXR activators in the small intestine. Biochem. Biophys. Res. Commun. 371(4):675–678.
Iseki S.., Kanda T.., Hitomi M.., Ono T.1991. Ontogenic appearance of three fatty acid binding proteins in the rat stomach. Anat. Rec. 229(1):51–60.

Jiang S.Y.., Shen S.R.., Shyu R.Y.., Yu J.C.., Harn H.J.., Yeh M.Y.., Lee M.M.., Chang Y.C.1999. ).Expression of nuclear retinoid receptors in normal, premalignant and malignant gastric tissues determined by in situ hybridization. Br. J. Cancer. 80(1-2):206–214.

Jiang Y.Z.., Li X.W.., Yang G.X.2006. Sequence characterization, tissue-specific expression and polymorphism of the porcine (Sus scrofa) liver-type fatty acid binding protein gene. Yi Chuan Xue Bao. 33(7):598–606.
Karam S.M.., Hassan W.M.., John R.2005. Expression of retinoid receptors in multiple cell lineages in the gastric mucosae of mice and humans. J. Gastroenterol. Hepatol. 20(12):1892–1899.

Kearney K.E.., Pretlow T.G.., Pretlow T.P.2009. Increased expression of fatty acid synthase in human aberrant crypt foci: possible target for colorectal cancer prevention. Int. J. Cancer. 125(1):249–252.

Kusakabe T.., Nashimoto A.., Honma K.., Suzuki T.2002. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 40(1):71–79.

Laryea M.., Watt K.D.., Molinari M.., Walsh M.J.., McAlister V.C.., Marotta P.J.., Nashan B.., Peltekian K.M.2007. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 13(8):1109–1114.

MacKenzie D.A.., Hullett D.A.., Sollinger H.W.2003. Xenogeneic transplantation of porcine islets: an overview. Transplantation. 76(6):887–891.

Mildner A.M.., Clarke S.D.1991. Porcine fatty acid synthase: cloning of a complementary DNA, tissue distribution of its mRNA and suppression of expression by somatotropin and dietary protein. J. Nutr. 121(6):900–907.

Mollevi D.G.., Jaurrieta E.., Ribas Y.., Hurtado I.., Serrano T.., Gomez N.., de Oca J.., Fiol C.., Figueras J.2000. Liver xenotransplantation: changes in lipid and lipoprotein concentration after longterm graft survival. J. Hepatol. 32(4):655–660.
Monbaliu D.., de Vries B.., Crabbe T.., van Heurn E.., Verwaest C.., Roskams T.., Fevery J.., Pirenne J.., Buurman W.A.2005. Liver fatty acid-binding protein: an early and sensitive plasma marker of hepatocellular damage and a reliable predictor of graft viability after liver transplantation from non-heart-beating donors. Transplant. Proc. 37(1):413–416.

Murthy S.., Tong H.., Hohl R. J.2005. Regulation of fatty acid synthesis by farnesyl pyrophosphate. J. Biol. Chem. 280(51):41793–41804.

Nomiyama T.., Bruemmer D.2008. Liver X receptors as therapeutic targets in metabolism and atherosclerosis. Curr. Atheroscler. Rep. 10(1):88–95.

Pakarinen M.P.., Kuusanmaki P.., Lauronen J.., Paavonen T.., Halttunen J.2003. ).Effects of ileum transplantation and chronic rejection on absorption and synthesis of cholesterol in pigs. Pediatr. Surg. Int. 19(9-10):656–661.

Portilla, D.,. Dent C.., Sugaya T.., Nagothu K. K.., Kundi I.., Moore P.., Noiri E.., Devarajan P.2008. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 73(4):465–472.
Ronnett G. V.., Kleman A. M.., Kim E. K.., Landree L. E.., Tu Y.2006. Fatty acid metabolism, the central nervous system, and feeding. Obesity. 14(5):201S–207S.

Roth K.A.., Cohn S.M.., Rubin D.C.., Trahair J.F.., Neutra M.R.., Gordon J.I.1992. Regulation of gene expression in gastric epithelial cell populations of fetal, neonatal, and adult transgenic mice. Am. J. Physiol. 263, G186-G197.
Sato T.., Yamamoto H.., Sawada N.., Nashiki K.., Tsuji M.., Muto K.., Kume H.., Sasaki H.., Arai H.., Nikawa T.., Taketani Y.., Takeda E.2006. Restraint stress alters the duodenal expression of genes important for lipid metabolism in rat. Toxicology. 227(3):248–261.

Scheepe J.R.., van den Hoek J.., Junemann K.P.., Alken P.2007. A standardised mini pig model for in vivo investigations of anticholinergic effects on bladder function and salivation. Pharmacol. Res. 55(5):450–454.

Schroeder F.., Petrescu A.D.., Huang H.., Atshaves B.P.., McIntosh A.L.., Martin G. G.., Hostetler H. A.., Vespa A.., Landrock D.., Landrock K.K.., Payne H.R.., Kier A.B.2008. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 43(1):1–17.

Storch J.., Corsico B.2008. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 28:73–95.

van der Veen J.N.., van Dijk T.H.., Vrins C.L.., van Meer H.., Havinga R.., Bijsterveld K.., Tietge U.J.., Groen A.K.., Kuipers F.2009. Activation of the liver X-receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284(29):19211–19219.
Wang Y.D.., Chen W.D.., Moore D.D.., Huang W.2008. FXR: a metabolic regulator and cell protector. Cell Res. 18(11):1087–1095.

Go to : 
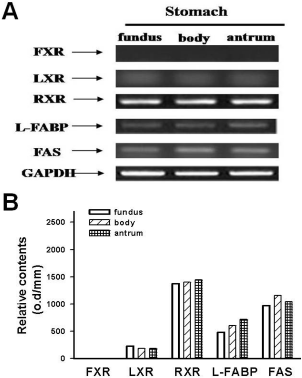 | Figure 1.The expression of FXR, LXR, RXR-beta, L-FABP, and FAS mRNA in the fundus, body and antrum of stomach in miniature pigs (A). mRNA expression was evaluated by RT-PCR as described in Materials and Methods nsection. Beta-actin was used as an endogenous control. Lower panel depicts the relative contents of optimal density in stomach (B). |
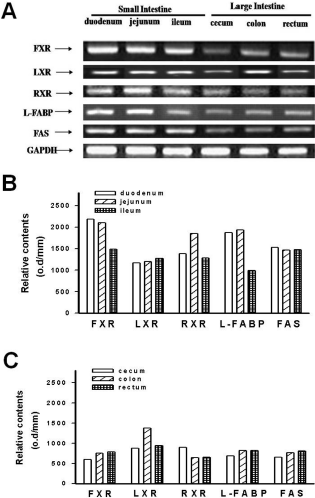 | Figure 2.The expression of FXR, LXR, RXR-beta, L-FABP, and FAS mRNA in the small intestine (duodenum, jejunum and ileum) and large intestine (cecum, colon and rectum) in miniature pigs (A). Lower panel depicts the relative contents of optimal density in small intestine (B) and large intestine (C). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download