Abstract
In order to assess the microbiological contamination of laboratory mice and rats in Korea over the 2-year period from 2007 to 2008, we monitored animals housed in mouse and rat facilities equipped with barrier systems. In a barrier animal facility in Korea, the most important viruses in the identified pathogen were the mouse hepatitis virus (MHV) and Pasteurella (Pa.) pneumotropica, and Staphylococcus aureus was identified as the most common bacterial pathogen in Korea. The most commonly detected parasite in the identified pathogen was Trichomonas spp. in the mouse facilities and Entamoeba spp. in the rat facilities. In many cases, these pathogen-contaminated animals were genetically modified animals obtained from the university. Currently, consistent with the increased transfer of genetically modified animals between domestic and foreign animal facilities, the Pa. pneumotropica and parasites infection rates were shown to have increased as compared to those of the 2004-2006 period. Indeed, the MHV infection rate has been maintained at almost 20% in Korean animal facilities over the past 10 years. These results showed that effective quarantine programs for contaminated genetically engineered mutant mice and the monitoring of regular or irregular MHV monitoring in animal colonies should help to reduce pathogen contamination in Korean animal facilities.
REFERENCES
Baker D.G.1998. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin. Microbiol. Rev. 11:231–266.

Fox J.G.., Lee A.1997. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab. Anim. Sci. 47:222–255.
Fox J.G.., Dewhirst F.E.., Tully J.G.., Paster B.J.., Yan L.., Taylor N.S.., Collins M.J.Jr.., Gorelick P.L.., Ward J.M.1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 32:1238–1245.
Hayashimoto N.., Aiba T.., Itoh K.., Kato M.., Kawamoto E.., Kiyokawa S.., Morichika Y.., Muraguchi T.., Narita T.., Okajima Y.., Takakura A.., Itoh T.2005. Identification procedure for Pasteurella pneumotropica in microbiologic monitoring of laboratory animals. Exp. Anim. 54:123–129.

Hsu C.C.., Wobus C.E.., Steffen E.K.., Riley L.K.., Livingston R.S.2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 12:1145–1151.

Itoh T.1999. Quality testing system for SPF animals in Japan and problems in the management of such systems. In: Microbial and Phenotypic Definition of Rats and Mice, pp. 15-23, National Academy Press, Washington DC.
Itoh T.2000. Emerging diseases in mice and rats. In: Microbial Status and Genetic Evaluation of Mice and Rats, pp. 40-43. National Academy Press, Washington DC.
Karst S.M.., Wobus C.E.., Lay M.., Davidson J.., Virgin H.W.2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 299:1575–1578.

Lee B.C.., Jang M.K.., Chae K.R.., Hwang D.Y.., Kim B.G.., Jee S.W.., Shim S.B.., Lee S.H.., Sin J.S.., Bae C.J.., Woo J.M.., Cho J.S.., Joo K.S.., Kim C. K.2008. Survey on the current status of laboratory animal quality control program in Korea. Lab. Anim. Res. 24:9–17.
Mahler M.., Nicklas W.2004. Health monitoring. In The laboratory mouse (Hendrich, H.J.), pp.449–462. Elsevier academic press, London.
Rehg J.E.., Toth L.A.1998. Rodent quarantine programs: purpose, principles, and practice. Lab. Anim. Sci. 48:438–447.
Shim S.B.., Hwang D.Y.., Kim C.K.., Kim Y.K.., Jee S.W.., Lee S.H.., Hwang Y.M.., Lee S.H.., Seo, S.J. Kang H.G.., Cho J.S.2006. Survey on current status of laboratory animals for establishing new national policy. Lab. Anim. Res. 22:55–60.
Waggie K.., Kagiyama N.., Allen M.A.., Nomura T.1994. Manual of Microbiologic Monitoring of Laboratory Animals, 2nd ed., NIH Publication, Maryland.
Won Y.S.., Jeong E.S.., Park H.J.., Lee C.H.., Nam K.H.., Kim H.C.., Hyun B.H.., Lee S.K.., Choi Y.K.2006. Microbiological contamination of laboratory mice and rats in Korea from 1999 to 2003. Exp. Anim. 55:11–6.

Won Y.S.., Kwon H.J.., Choi Y.K.., Kim S.W.., Lee H.J.., Koo B.C.., Lee S.B.., Nam K.H.., Moon O.S.., Jeong E.S.., Kim H.C.2007. Microbiological contamination of laboratory mice and rats in Korea from 2004 to 2006. Lab. Anim. Res. 23:327–332.
Yamamoto H.., Sato H.., Yagami K.., Arikawa J.., Furuya M.., Kurosawa T.., Mannen K.., Matsubayashi K.., Nishimune Y.., Shibahara T.., Ueda T.., Itoh T.2001. Microbiological contamination in genetically modified animals and proposals for a microbiological test standard for national universities in Japan. Exp. Anim. 50:397–407.

Figure 1.
The microbiological contamination in the mouse breeding facilities. The indicated values shown are prevalence in animal facilities. Several pathogens not listed in Figure 1 were not isolated in this study. A: The prevalence of bacteria, virus and parasite in mouse facilities, B: The prevalence of intestinal parasites in mouse facilities.
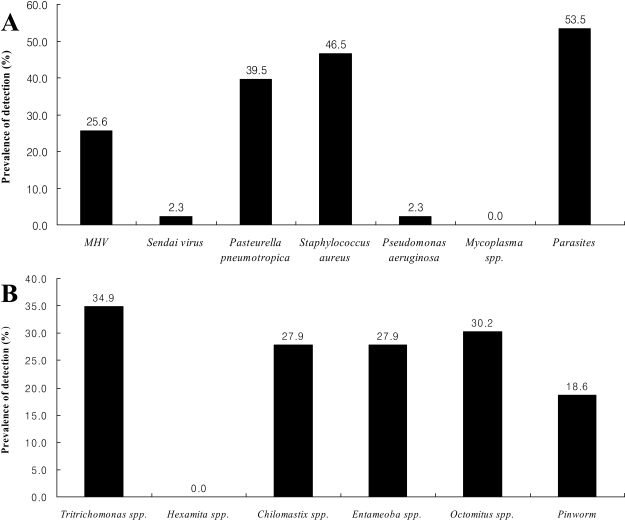
Figure 2.
The microbiological contamination in the rat breeding facilities. The indicated values shown are prevalence in animal facilities. Several pathogens not listed in Figure 2 were not isolated in this study. A: The prevalence of bacteria, virus and parasite in rat facilities, B: The prevalence of intestinal parasites in rat facilities.
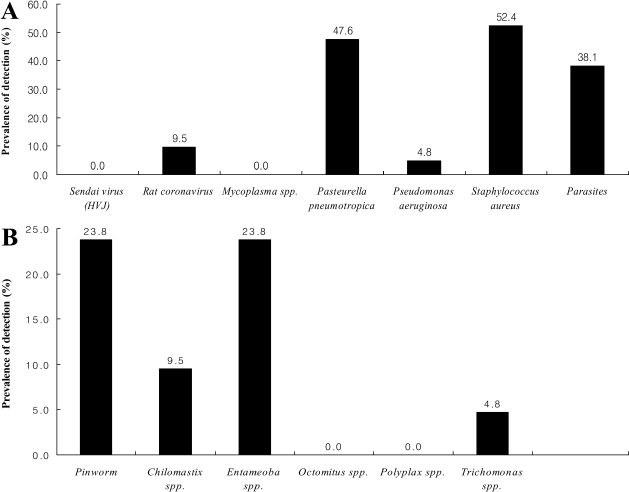
Table 1.
Number of organizations and animals investigated in this study
| Classification | Mouse | Rat | ||
|---|---|---|---|---|
| No. of organizations | No. of animals | No. of organizations | No. of animals | |
| Company | 7 | 307 | 5 | 58 |
| University | 31 | 2,819 | 14 | 334 |
| Research institute | 5 | 477 | 2 | 155 |
| Total | 43 | 3,603 | 21 | 547 |
Table 2.
Selected pathogen list for microbiological monitoring in this study




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download