Abstract
The present study was carried out to provide a guideline for injecting tribromoethanol (TBE) as the main anesthetic agent, while adjusting the doses of xylazine (X) and medetomidine (M) according to different strains of mice (male ICR, C57BL/6, and BALB/c). Seven intraperitoneal injection anesthesia protocols using TBE and mixtures of TBE and α2–adrenergic agonists (TBE/X and TBE/M) were compared in terms of their efficacy and safety (anesthetic duration, death rate, and the development of pathological lesions of abdominal organs). All animals that were injected with a low dose of TBE (200 mg/kg) displayed clear signs of light anesthesia with a strong pedal withdrawal reflex. Despite the good anesthetic effect, a high dose of TBE (400 mg/kg) was not a suitable anesthetic for major surgery in all mouse strains because of the risk of pathologic changes in the abdominal organs, such as retention of the digestive tract, peritonitis, and fibrinoid adhesion. TBE200/X10 and TBE200/M0.5 (TBE, 200 mg/kg; X, 10 mg/kg; M, 0.5 mg/kg) appeared to be safe and provided satisfactory anesthesia in ICR mice. Finally, there were clear differences in anesthetic efficacy among ICR, C57BL/6, and BALB/c strains. TBE/M and TBE/X did not anesthetize BALB/c mice, and it anesthetized C57BL/6 mice for a short time. When administered with TBE/X and TBE/M maintained the sedation of ICR mice. We were able to establish different regimes for each strain (TBE200/X20 for C57BL/6, TBE300/X10 and TBE200/M1 for BALB/c). Our results showed that TBE/X and TBE/M could be recommended as an anesthetic mixture, with the dose appropriately adjusted according to mouse strain.
Go to : 
REFERENCES
Arras M.., Autenried P.., Rettich A.., Spaeni D.., Rulicke T.2001. Optimization of intraperitoneal injection anesthesia in mice: Drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 51(5):443–456.
Bette M.., Schlimme S.., Mutters R.., Menendez S.., Hoffmann S.., Schulz S.2004. Influence of different anaesthetics on proinflammatory cytokine expression in rat spleen. Lab. Anim. 38(3):272–279.
Buitrago S.., Martin T.E.., Tetens-Woodring J.., Belicha-Villanueva A.., Wilding G.E.2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J. Am. Assoc. Lab. Anim. Sci. 47(1):11–17.
Coria-Avila G.A.., Gavrila A.M.., Menard S.., Ismail N.., Pfaus J.G.2007. Cecum location in rats and the implications for intraperitoneal injections. Lab. Anim. (NY). 36(7):25–30.

Fish R.E.., Meyer R.E.2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers. In Anesthesia and Analgesia in Laboratory Animals (Fish, R.E. ed.), 2nd ed., pp. 27-82, Academic Press, Burlington.
Flecknell P.A.2005. Laboratory Animal Anaesthesia, pp. 160-171, Elsevier Academic Press, San Diego.
Gardner D.J.., Davis J.A.., Weina P.J.., Theune B.1995. Comparison of tribromoethanol, ketamine/acetylpromazine, telazol/xylazine, pentobarbital, and methoxyflurane anesthesia in HSD: ICR mice. Lab. Anim. Sci. 45(2):199–204.
Gopalan C.., Hegade G.M.., Bay T.N.., Brown S.R.., Talcott M.R.2005. Tribromoethanol-medetomidine combination provides a safe and reversible anesthetic effect in Sprague-Dawley rats. Contemp. Top Lab. Anim. Sci. 44(1):7–10.
Green C.J.1975. Neuroleptanalgesic drug combinations in the anaesthetic management of small laboratory animals. Lab. Anim. 9(3):161–178.

Hall J.C.., Heel K.A.., Papadimitriou J.M.., Platell C.1998. The pathobiology of peritonitis. Gastroenterology. 114(1):185–196.

Hedenqvist P.., Hellebrekers, L.J. and Van Hoosier G.L.2003. Laboratory animal analgesia, anesthesia, and euthanasia. In Handbook of Laboratory Animal Science (Hau, J. ed.), vol. I, 2nd ed., pp. 413-457, CRC Press, Boca Raton.
Hedrich H.J.., Bullock G.R.2004. The Laboratory Mouse, pp. 543-554, Elsevier Academic Press, San Diego.
Kiatchoosakun S.., Kirkpatrick D.., Hoit B.D.2001. Effects of tribromoethanol anesthesia on echocardiographic assessment of left ventricular function in mice. Comp. Med. 51(1):26–29.
Lieggi C.C.., Artwohl J.E.., Leszczynski J.K.., Rodriguez N.A.., Fickbohm B.L.., Fortman J.D.2005a. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp. Top Lab. Anim. Sci. 44(1):17–22.
Lieggi C.C.., Fortman J.D.., Kleps R.A.., Sethi V.., Anderson J.A.., Brown C.E.., Artwohl J.E.2005b. An evaluation of preparation methods and storage conditions of tribromoethanol. Contemp. Top Lab. Anim. Sci. 44(1):11–16.
Mascia M.P.., Machu T.K.., Harris R.A.1996. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br. J. Pharmacol. 119(7):1331–1336.

National Research Council. 1996. Guide for the Care and Use of Laboratory Animals, pp. 56-70, National Academy Press, Washington DC.
Reid W.C.., Carmichael K.P.., Srinivas S.., Bryant J.L.1999. Pathologic changes associated with use of tribromoethanol (avertin) in the Sprague Dawley rat. Lab. Anim. Sci. 49(6):665–667.
Tarin D.., Sturdee A.1972. Surgical anaesthesia of mice: Evaluation of tribromoethanol, ether, halothane and methoxyflurane and development of a reliable technique. Lab. Anim. 6(1):79–84.

Thompson J.S.., Brown S.A.., Khurdayan V.., Zeynalzadedan A.., Sullivan P.G.., Scheff S.W.2002. Early effects of tribromoethanol, ketamine/xylazine, pentobarbitol, and isoflurane anesthesia on hepatic and lymphoid tissue in ICR mice. Comp. Med. 52(1):63–67.
Ticku M.K.., Kulkarni S.K.1988. Molecular interactions of ethanol with GABAergic system and potential of RO15-4513 as an ethanol antagonist. Pharmacol. Biochem. Behav. 30(2):501–510.

van Goor H.., de Graaf J.S.., Grond J.., Sluiter W.J.., van der Meer J.., Bom V.J.., Bleichrodt R.P.1994. Fibrinolytic activity in the abdominal cavity of rats with faecal peritonitis. Br. J. Surg. 81(7):1046–1049.

Go to : 
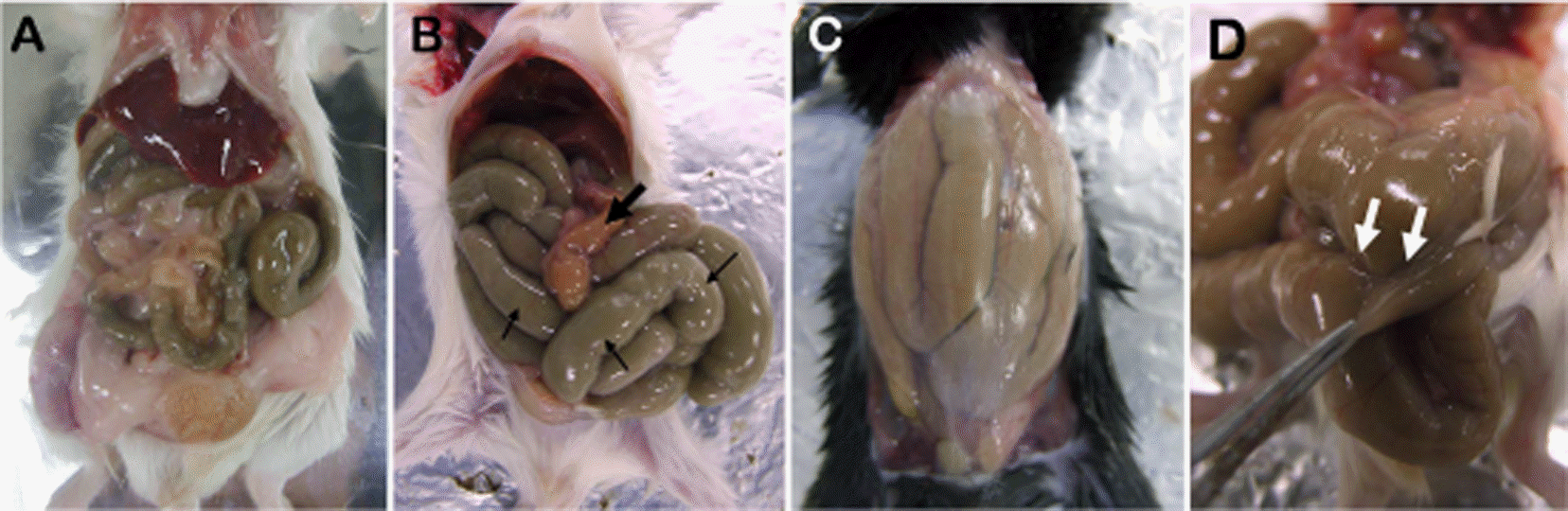 | Figure 1.Appearance of a mouse's abdominal cavity at 10 dpi. (A) Gross necropsy of ICR mice that received saline intraperitoneally. Normal appearance of the abdominal cavity is noted. (B) Gross necropsy of ICR mouse that received 400 mg/kg tribromoethanol (TBE) intraperitoneally. The stomach is grossly empty (thick, dark arrow). The intestine is distended with fluid and digested food, supporting a gross description of ileus (thin, dark arrow). (C) Thin abdominal wall and distension of the gastrointestinal tract found upon gross necropsy of C57BL/6 mouse that was administered 400 mg/kg TBE. (D) BALB/c mouse that received 400 mg/kg TBE showed focally extensive area of fibrous adhesion between the small intestine and cecum, which were filled with digested food (open arrow). |
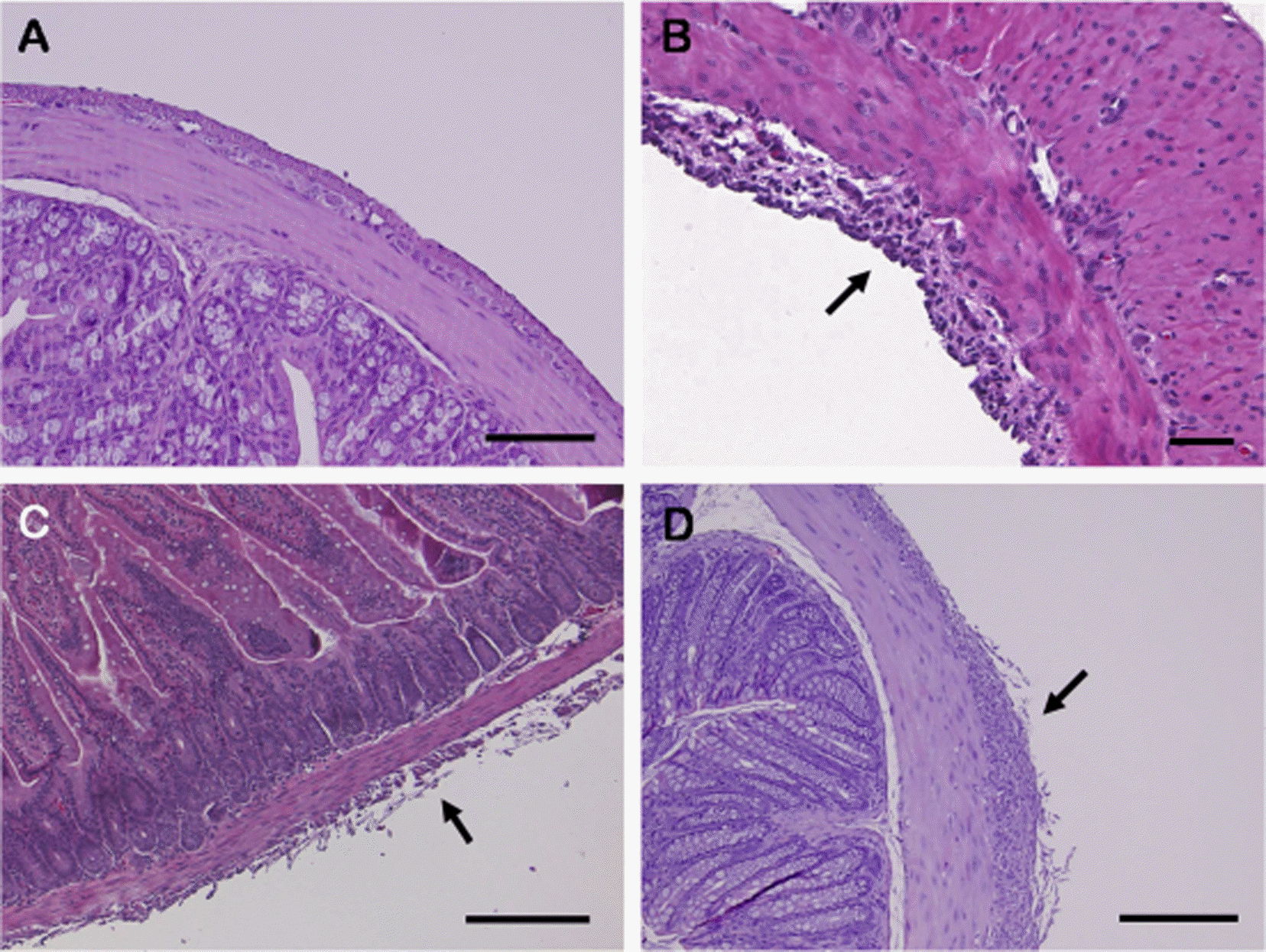 | Figure 2.Microscopic photographs of gastrointestinal serosa in mice at 10 dpi. (A) Normal histology of large intestine from ICR mouse that was injected with saline intraperitoneally (bar, 100 µm). (B) Histopathology of a stomach from ICR mouse that received 400 mg/kg tribromoethanol (TBE) intraperitoneally. Section demonstrates intermixed mononuclear cells with the fibrous tissue in the serosa (arrow). Hematoxylin and eosin stain; bar, 50 µm (C) Histopathology of the large intestine from a C57BL/6 mouse that received 400 mg/kg TBE intraperitoneally. The section demonstrates fibrosis and inflammation of the serosa (arrow). Hematoxylin and eosin stain; bar, 200 µm. (D) Histopathology of the large intestine from a BALB/c mouse that was administered 400 mg/kg TBE intraperitoneally. This large intestine section demonstrates mononuclear cells intermixed with the fibrous tissue (arrow). Hematoxylin and eosin stain; bar, 200 µm. |
Table 1.
Guidelines for tribromoethanol anesthesia in several facilities or literatures in mice
| Dose∗ (mg/kg) | References |
|---|---|
| • NIH Anesthesia/Analgesia formulary | |
| • Cornell Center for Animal Resources and Education, in Cornell University | |
| • Animal Care Facility in Laurentian University | |
| 125-250 | • Unit for Laboratory Animal Medicine in University of Michigan Medical School |
| • Laboratory Animal Research Center in Samsung Biomedical Research Institute, Korea | |
| • Laboratory animal research center in Chungbuk National university, Korea | |
| 125-300 | • (Flecknell, 2005) |
| • (Fish et al., 2008) | |
| 125-400 | • Safety Services in University of California, Davis |
| 225-240 | • Research Animal Resources in University of Minnesota |
| • Guidelines in Duke University and Medical Center | |
| 250 | • Guidelines in University of Rochestar Medical Center |
| • (Hedenqvist et al., 2003) | |
| 250-500 | • IACUC in University of California |
| 300-600 | • Animal Resource Center in Case Western Reserve University |
| 310 | • IACUC in University of Tennessee |
| 360-800 | • Guidelines in Baylor College of Medicine |
| 400-600 | • Office of the Campus Veterinarian in Washington State university |
| 400-750 | • Veterinary Services in Florida Atlantic University |
Table 2.
Summary of experiments and number of animals used
Table 3.
Efficacy and safety of anesthesia after administration of each different regimes
| P2a | P3b | P4c | P5d | P6e | P7f | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICR | C57BL/6 | BALB/c | ICR | C57BL/6 | BALB/c | ICR | C57BL/6 | BALB/c | C57BL/6 | BALB/c | BALB/c | ||
| ICR | C57BL/6 | BALB/c | ICR | C57BL/6 | BALB/c | ICR | C57BL/6 | BALB/c | C57BL/6 | BALB/c | BALB/c | ||
| ANES rate (n/n) | 10/10 | 10/10 | 4/10 | 10/10 | 10/10 | 0/10 | 8/10 | 10/10 | 7/10 | 10/10 | 8/10 | 10/10 | |
| INDg | 01:21 | 01:39 | 03:51 | 02:53 | 03:18 | NA | 02:54 | 02:04 | 07:10 | 03:23 | 03:30 | 04:16 | |
| ±00:07 | ±00:05 | ±00:26∗† | ±00:32 | ±00:36 | ±00:46 | ±00:16 | ±00:41∗† | † ±00:19 | ±00:23 | ±00:25 | |||
| Mean ±SEM (mm:ss) | ANESh | 39:05 | 31:02 | 08:48 | 39:28 | 13:26 | NA | 65:38 | 73:59 | 17:39 | 27:03 | 59:15 | 24:43 |
| ±03:01 | ±03:31 | ±01:24∗† | ±05:08 | ±00:56∗ | ±06:40 | ±07:34∗ | ±04:06∗† | † ±04:28 | ±06:37 | ±02:53 | |||
| RECi | 26:45 | 51:31 | 27:29 | 09:13 | 18:49 | NAj | 48:28 | 42:26 | 73:04 | 23:34 | 41:31 | 57:35 | |
| ±04:41 | ±02:54∗ | ±04:47† | ±03:00 | ±02:12∗ | ±08:51 | ±05:21∗ | ±05:31† | ±02:38 | ±06:23 | ±05:58 | |||
| Death rate | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 2/10 | 0/10 | 0/10 | 0/10 | 2/10 | 0/10 | |
| Gross lesion | 1 dpik | 2/5 | 5/5 | 5/5 | 0/5 | 0/5 | 0/5 | 0/4 | 0/5 | 0/5 | 0/5 | 0/4 | 0/5 |
| 10 dpi | 4/5 | 4/5 | 4/5 | 0/5 | 0/5 | 0/5 | 0/4 | 0/5 | 0/5 | 0/5 | 0/4 | 0/5 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download