Abstract
Spinal cord injury often leads to central neuropathic pain syndromes, such as allodynic and hyperalgesic behaviors. Electrophysiologically, spinal dorsal horn neurons show enhanced activity to non-noxious and noxious stimuli as well as increased spontaneous activity following spinal cord injury, which often called hyperexcitability or central sensitization. Under hyperexcitable states, spinal neurons lose their ability of discrimination and encoding somatosensory information followed by abnormal somatosensory recognition to non-noxious and noxious stimuli. In the present review, we summarize a variety of pathophysiological mechanisms of neuronal hyperexcitability for treating or preventing central neuropathic pain syndrome following spinal cord injury.
Go to : 
REFERENCES
Bennett A.D.., Everhart A.W.., Hulsebosch C.E.2000. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 859:72–82.

Brambilla R.., Hurtado A.., Persaud T.., Esham K.., Pearse D.D.., Oudega M.., Bethea J.R.2009. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J. Neurochem. 110:765–778.
Carlton S.M.., Du J.., Tan H.Y.., Nesic O.., Hargett G.L.., Bopp A.C.., Yamani A.., Lin Q.., Willis W.D.., Hulsebosch, Hulsebosch, , Willis, W.D. and Hulsebosch C.E.2009. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 147:265–276.

Christensen M.D.., Hulsebosch C.E.1997. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 147:463–475.

Chu K.L.., Faltynek C.R.., Jarvis M.F.., McGaraughty S.2004. Increased WDR spontaneous activity and receptive field size in rats following a neuropathic or inflammatory injury: implications for mechanical sensitivity. Neurosci. Lett. 372:123–126.

123-126. Chung J.M.., Surmeier D.J.., Lee D.J.., Sorkin L.S.., Honda C.N.., Tsong Y.., Willis W.D.1986. Classification of primate spinothalamic and somatosensory thalamic neurons based on cluster analysis. J. Neurophysiol. 56:308–327.
Crown E.D.., Gwak Y.S.., Ye Z.., Johnson K.M.., Hulsebosch C.E.2008. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 213:257–267.

Crown E.D.., Ye Z.., Johnson K.M.., Xu G.Y.., McAdoo D.J.., Hulsebosch C.E.2006. Increases in the activated forms of ERK 1/2, p38MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 199:397–407.
Dougherty K.J.., Hochman S.2008. Spinal cord injury causes plasticity in a subpopulation of lamina ⅠGABAergic interneurons. J. Neurophysiol. 100:212–223.
Dougherty P.M.., Willis W.D.1991. Enhancement of spinothalamic neuron responses to chemical and mechanical stimuli following combined micro-ionotophoretic application of N-methyl-D-aspartic acid and substance P. Pain. 47:85–93.
Drew G.M.., Siddall P.J.., Duggan A.W.2001. Responses of spinal neurons to cutaneous and dorsal root stimuli in rats with mechanical allodynia after contusive spinal cord injury. Brain Res. 893:59–69.
Erschbamer M.., Pernold K.., Olson. L.J.2007. Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. Neuroscience. 27:6428–6435.

Genovese T.., Esposito E.., Mazzon E.., Di Paola R.., Meli R.., Bramanti P.., Piomelli D.., Calignano A.., Cuzzocrea S.2008. Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J. Pharmacol. Exp. Ther. 326:12–23.

Gwak Y.S.., Crown E.D.., Unabia G.C.., Hulsebosch C.E.2008. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 138:410–422.

Gwak Y.S.., Hulsebosch C.E.2009. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 161:895–903.

Gwak Y.S.., Hulsebosch C.E.2005. Upregulation of groupⅠmetabotropic glutamate receptors in neurons and astrocytes in the dorsal horn following spinal cord injury. Exp. Neurol. 195:236–243.
195, 236-243. Gwak Y.S.., Kim H.K.., Kim H.Y.., Leem J.W.2010. Bilateral hyperexcitability of thalamic VPL neurons following unilateral spinal injury in rats. J. Physiol. Sci. 60-. 59–66.
Gwak Y.S.., Tan H.Y.., Nam T.S.., Paik K.S.., Hulsebosch C.E.., Leem J.W.2006. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. 23:1111–1124.

Gwak Y.S.., Nam T.S.., Paik K.S.., Hulsebosch C.E.., Leem J.W.2003. ).Attenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the rat. Neurosci. Lett. 336:117–120.

Hains B.C.., Klein J.P.., Saab C.Y.., Craner M.J.., Black J.A.., Waxman S.G.2003. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 23:8881–8892.

Hains B.C.., Waxman S.G.2006. Activated microglia contribute to maintenance of chronic pain after spinal cord injury. J. Neurosci. 26:4308–4317.
Hou S.., Duale H.., Rabchevsky A.G.2009. Intraspinal sprouting of unmyelinated pelvic afferents after complete spinal cord injury is correlated with autonomic dysreflexia induced by visceral pain. Neuroscience. 159:369–379.

Christensen M.D.., Hulsebosch C.E.1997. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 147:463–475.

Iaci J.F.., Vecchione A.M.., Zimber M.P.., Caggiano A.O.2007. Chondroitin sulfate proteoglycans in spinal cord contusion injury and the effects of chondroitinase treatment. J. Neurotrauma. 24:1743–1759.

Inoue M.., Ma L.., Aoki J.., Ueda H.2008. Simultaneous stimulation of spinal NK1 and NMDA receptors produces LPC which unergoes ATX-mediated conversion to LPA, an initiator of neuropathic pain. J. Neurochem. 107:1556–1565.
Ji R.R.., Kohno T.., Moore K.A.., Woolf C.J.2003. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26:696–705.

Ji R.R.., Strichartz G.2004. Cell signaling and the genesis of neuropathic pain. Sci. STKE 2004. (252):reE14.
Kawasaki Y.., Zhang L.., Cheng J.K.., Ji R.R.2008. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 28:5189–5194.
Kim J.., Jung J.I.., Na H.S.., Hong S.K.., Yoon Y.W.2003. Effects of morphine on mechanical allodynia in a rat model of central neuropathic pain. Neuroreport. 14:1017–1020.

Labombarda F.., Coronel M.F.., Villar M.J.., Nicola A.F.., Gonzalez S.L.2008. Neuropathic pain and temporal expression of preprodynorphin, protein kinase C and N-methyl-D-aspartate receptor subunits after spinal cord injury. Neurosci. Lett. 447:115–119.
Leem J.W.., Kim H.K.., Hulsebosch C.E.., Gwak Y.S.2010. Ionotropic glutamate receptors contribute to maintained neuronal hyperexcitability following spinal cord injury in rats. Exp. Neurol. 224:321–324.

Li J.., McRoberts J.A.., Nie J.., Ennes H.S.., Mayer E.A.2004. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 109:443–452.
Lin Z.P.., Zhu Y.L.., Johnson D.R.., Rice K.P.., Nottoli T.., Hains B.C.., McGrath J.., Waxman S.G.., Sartorelli A.C.2008. Disruption of Camp and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Mol. Pharmacol. 73:243–251.
Liu J.., Wolfe D.., Hao S.., Huang S.., Glorioso J.C.., Mata M.., Fink. D.J.2004. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol. Ther. 10:57–66.

Liu S.., Xu G.Y.., Johnson K.M.., Echetebu C.., Ye Z.S.., Hulsebosch C.E.., McAdoo D.J.2008. Regulation of interleukin-1beta by the interleukin-1 receptor antagonist in the glutamate-injured spinal cord: endogenous neuroprotection. Brain Res. 1231:63–74.
McAdoo D.J.., Hughes M.G.., Nie L.., Shah B.., Clifton C.., Fullwood S.., Hulsebosch C.E.2005. The effect of glutamate receptor blockers on glutamate release following spinal cord injury. Lack of evidence for an ongoing feedback cascade of damage→glutamate release→damage→glutamate release→etc. Brain Res. 1038:92–99.
Merskey H.., Bogduk D.1994. Classification of Chronic Pain, IASP Press, Seattle.
Shim B.., Kim D.W.., Kim B.H.., Nam T.S.., Leem J.W.., Chung J.M.2005. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 132:193–201.

Siddall P.J.., Taylor D.A.., Cousins M.J.1997. Classification of pain following spinal cord injury. Spinal Cord. 35:69–75.

Willis W.D.., Coggeshall R.E.1991. Sensory Mechanism of the Spinal Cord, 2nd ed., Plenum Press, New York.
Willis W.D.., Westland K.N.1997. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 14:2–31.

Wu W.P.., Hao J.X.., Ongini E.., Impagnatiello F.., Presotto C.., Wiesenfeld-Hallin Z.., Xu X.J.2004. A nitric oxide (NO)-releasing derivative of gabapentin, NCX8001, alleviates neuropathic pain-like behavior after spinal cord and peripheral nerve injury. Br. J. Pharmacol. 141:65–74.
Go to : 
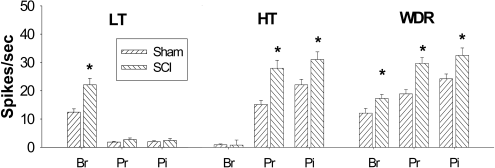 | Figure 1.Hyperexcitability of lumbar spinal dorsal horn neurons following low thoracic spinal cord injury. All phenotypes of lumbar spinal dorsal horn neurons showed significantly increased evoked activity in response to mechanical stimuli applied on the receptive field compared to sham controls. Low threshold (LT) neurons showed significant increase of brush evoked activity whereas high threshold (HT) neurons showed significantly increased activity to pressure and pinch stimuli, respectively. Wide dynamic range (WDR) neurons showed significantly increased activity to all three different mechanical stimuli. Br: brush, Pr: pressure, Pi: pinch stimuli. ∗P<0.05. |
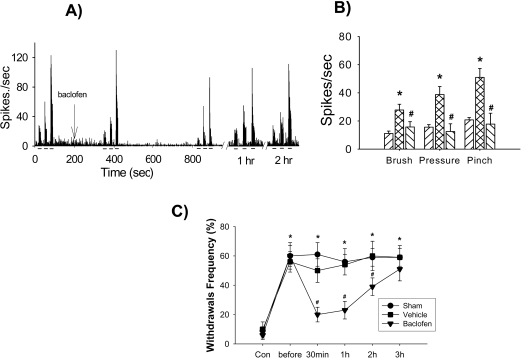 | Figure 2.Attenuation of neuronal hyperexcitability and mechanical allodynia by γ-aminobutyric acid (GABA) receptor agonist. (A) shows typical histograms of wide dynamic range (WDR) neurons in response to brush, pressure and pinch stimuli applied on the peripheral receptive field. (B) shows attenuation of WDR activity by topical application of baclofen (GABAB receptor agonist) in the lumbar spinal dorsal horn following low thoracic spinal cord injury. (C) shows attenuation of mechanical allodynia by intrathecal application of baclofen following spinal cord injury. ∗P<0.05 compared to control values; #P<0.05 compared to pre-drug (before) values. Modified from Gwak et al. (2006). |
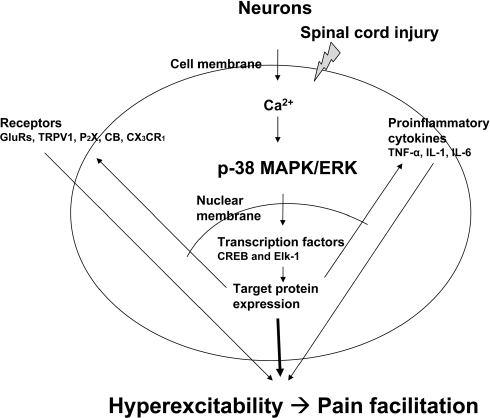 | Figure 3.Intracellular signaling pathway for neuronal hyperexcitability. Following spinal cord injury, massive influx of calcium ions through calcium-permeable channels into cytoplasm initiates activation of mitogen-associated protein kinase (MAPK) family followed by activation of transcription factors, which result in altered target protein expression, such as phosphorylation of receptors and ion channels in the membrane. Activation of ion channels and receptors directly contributed to neuronal hyperexcitability. This positive feedback maintains persistent hyperexcitability of spinal dorsal horn neurons following spinal cord injury. |
Table 1.
Proportional changes of thalamic VPL neurons following spinal hemisection injury. The sham group showed higher incidence of low threshold (LT) neurons than both wide dynamic range (WDR) and high threshold (HT) neurons. After hemisection, both ipsilateral and contralateral sides of thalamic VPL neurons showed higher incidences of WDR neurons than LT neurons, respectively. No HT neurons were observed in the rats with spinal cord injury (hemisection). Modified from Gwak et al. (2010)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download