Abstract
Infection with Helicobacter pylori (H. pylori) is strongly associated with duodenal and gastric ulcers. Substantial epidemiological data has revealed that high rates of H. pylori infection might be related to high rates of gastric cancer and gastric adenocarcinoma. In this study, a medicinal herbal plant, Cinnamomum cassia, was examined and screened for anti-H. pylori activity. Seventy percent ethanol was used for herbal extraction. For anti-H. pylori activity screening, inhibitory zone tests as an in vitro assay and in vivo study using a Mongolian gerbil (Meriones unguiculatus) model were performed. Also, the safety of herbal compounds was evaluated by animal study. As a result of inhibitory zone test, Cinnamomum cassia extract demonstrated strong anti-H. pylori activities. Also, as results of in vivo animal studies, Cinnamomum cassia demonstrated strong therapeutic effects against H. pylori infection according to the criteria of histological examination and rapid urease test. As results of the safety study, after 28 days treatment of the Cinnamomum cassia extract, the animals were not detected any grossly and histological changes. These results demonstrate that it can be successfully cured against H. pylori infection and protected from H. pylori-induced pathology with Cinnamomum cassia. It could be a promising native herb treatment for patients with gastric complaints including gastric ulcer caused by H. pylori.
Go to : 
REFERENCES
Ahn Y.O.., Park B.J.., Yoo K.Y.., Kim N.K.., Heo D.S.., Lee J.K.., Ahn H.S.., Kang D.H.., Kim H.., Lee M.S.., Park T.S.1991. Incidence estimation of stomach cancer among Koreans. J. Kor. Med. Sci. 6:7–14.

Bamford K.B.., Fan X.., Crowe S.E.1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 114:482–492.

Eun C.S.., Han D.S.., Park J.Y.., Jeon Y.C.., Hahm J.S.., Kim K.S.., Kang J.O.2003. Changing pattern of antimicrobial resistance of Helicobacter pylori in Korean peptic ulcer diseases. J. Gastroenterol. 38:436–441.
Giordani R.., Regli P.., Kaloustian J.., Portugal H.2006. Potentiation of antifungal activity of amphotericin B by essential oil from Cinnamomum cassia. Phyother. Res. 20:58–61.
Hahm K.B.., Lee K.M.., Kim Y.B.., Han S.U.., Kim M.W.2001. Animal models of Helicobacter pylori infection. Korean J. Gastroenterol. 37:399–405.
Han D.S.2007. Treatment of H. pylori infection and current status of vaccine development. Hanyang Med. J. 27:81–95.
Hansson L.E.., Nyren O.., Hsing A.W.., Bergstrom R.., Josefsson S.., Chow W.H.., Fraumeni J.F.., Adami H.O.1996. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 355:242–249.

Harris A.1997. Treatment of Helicobacter pylori. Drugs In Today. 33:59–66.
Hibi K.., Mitomi H.., Koizumi W.., Tanabe S.., Saigenj K.., Okayasu I.1997. Enhanced cellular proliferation and p53 accumulation in gastric mucosa chronically infected with Helicobacter pylori. Am. J. Clin. Patho. 108:26–34.

Honda S.., Fujioka T.., Tokieda M.., Satoh R.., Nishizono A.., Nasu M.1998. Development of Helicobacter pylori induced-gastric carcinoma in Mongolian gerbils. Cancer Res. 58:4255–4259.
Ihan A.., Tepez B.., Gubina M.., Malovrh T.., Kopitar A.1999. Diminished interferon-ã production in gastric mucosa T lymphocytes after Helicobacter pylori eradication in duodenal ulcer patients. Hepatogastroemterology. 46:1740–1745.
Jung H.C.., Kim J.M.., Song I.S.., Kim C.Y.1997. Helicobacter pylori induces an array of proinflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8,-1α/β, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumor necrosis factor-α. J. Gastroenterol. Hepatol. 12:473–480.
Jung E.T.., Park M.Y.., Lee E.W.., Park U.Y.., Jang D.S.1998a. Antimicrobial characteristics against spoilage microorganisms and food preservative effect of Cinnamon (Cinnamomum cassis Blume) bark extract. J. Life Sci. 8:648–653.
Jung E.T.., Park M.Y.., Lee J.K.., Jang D.S.1998b. Anitimicrobial activity and antimutagenesis of Cinnamon (Cinnamomum cassia Blume) bark extract. J. Food Hygiene Safety. 13:337–343.
Jung T.S.., Kang S.C.., Choi Y.J.., Jeon B.S.., Park J.W.., Jung S.A.., Song J.Y.., Choi S.H.., Park S.G.., Choe M.Y.., Lee B.S.., Byun E.Y.., Baik S.C.., Lee W.K.., Cho M.J.., Youn H.S.., Ko G.H.., Rhee K.H.2000. Two-dimensional gel electrophoresis of Helicobacter pylori for proteomic analysis. J. Bacteriol. Virol. 35:97–108.
Kim B.W.., Choi M.G.., Choi H.., Moon S.B.., Kim B.K.., Chae H.S.., Kim J.K.., Chung I.S.., Chung K.W.., Sun H.S.., Park D.H.1999. Pooled analysis of antibiotic therapy for Helicobacter pylori eradication in Korea. Korean. J. Gastroenterol. 34:42–49.
Kim J.M.2007. Antibiotic resistance in Helicobacter pylori. Hanyang Medical Reviews. 27:80–95.
Kim J.M.., Kim J.S.., Jung H.C.., Song I.S.., Kim C.Y.2000. Apoptosis of human gastric epithelial cells via caspase-3 activation in response to Helicobacter pylori infection: possible involvement of neutrophils through tumor necrosis factor-alpha and soluble Fas ligands. Scand. J. Gastroenterol. 35:40–48.
Kim J.M.., Kim J.S.., Jung H.C.., Kim N.., Kim Y.J.., Song I.S.2004. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob. Agents Chemother. 48:4843–4847.
Koehn F.E.., Carter G.T.2005. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4:206–220.

Kunin C.M.1993. Resistance to antimicrobial drugs-a worldwide calamity. Ann. Intern. Med. 118:557–561.

Lee J.., Kim S.M.., Im E.H.., Choi Y.W.., Kim Y.M.., Kim P.S.., Lee J.H.2005. The prevalence of antimicrobial resistance in Helicobacter pylori isolated in Daejeon. Korean J. Clin. Microbiol. 8:47–50.
Lee J.U.., Kim S.H.., Park T.W.., Kim O.J.2006. Establishment of Ethanol-pretreation animal model to study Helicobacter pylori infection. Korean J. Vet. Res. 46:327–355.
Lee J.U.., Kim S.H.., Park T.W.., Kim O.J.2007. Elder ages decreases the susceptibility for Helicobacter pylori infection in an animal model. Korean J. Vet. Res. 47:77–84.
Lee J.Y.., Kim W.., Gawk G.Y.., Park S.C.., Ye B.D.., Lee S.H.., Kim S.G.., Kim J.S.., Jun H.C.., Song I.S.2002. Reinfection rate and clinical manifestation of Helicobacter pylori-positive peptic ulcer disease after triple therapy containing clarithromycin. Korean J. Gastrornterol. 39:93–100.
Lee K.H.., Choi E.M.2006. Stimulatory effects of extract prepared from the bark of Cinnamomum cassia blume on the function of osteoblastic MC3T3-E1 cells. Phytother. Res. 20:952–960.
Lim T.H.., Lee J.M.., Cha B.J.2002. Antifungal activity and identification of an Actinomycetes straun isolated from mummified peaches. Kor. J. Appl. Microbiol. Biotechnol. 28:161–166.
Lin C.C.., Wu S.J.., Chang C.H.., Ng L.T.2003. Antioxidant activity of Cinnamomum cassia. Phytother. Res. 17:726–730.
Marshall B.J.., Warren J.R.1984. Unidentified curved bacilli in the stomach of patients with gastric and peptic ulceration. Lancet. 1:1311–1315.
Mau J.., Chen C.., Hiseh P.2001. Antimicrobial effect of extracts from Chinese chive, cinnamon, and corni fructus. J. Agric. Food Chem. 49:183–188.

Park H.J.., Kim J.W.., Lee J.H.., Shin J.H.., Yu K.A.2000. Detection of clarithromycin resistant Helicobacter pylori by polymerase chain reaction. Korean J. Gastroenterol. 47:459–461.
Park G.H.., Go D.S.2001. Anti-allergic compound isolated from Cinnamomum cassia. J. Korean. Soc. Appli. Biol. Chem. 44:40–42.
Prakash D.., Suri S.., Upadhyay G.., Singh B.N.2007. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 58:18–28.

Rao C.V.., Vijayakumar M.2008. Effect of quercrtin, flavonoids and alpha-tocopherol, an antioxidant vitamin on experimental reflux oesophagitis in rats. Eur. J. Pharmacol. 589:233–238.
Shan B.E.., Yoshida Y.., Sugiura T.., Yamashita U.1999. Stimulating activity of Chinese medicinal herbs on human lymphocytes in vitro. Int. J. Immunopharmacol. 21:149–159.

Shim S.G.., Kim J.J.., Kim Y.H.., Sung I.K.., Son H.J.., Lee K.T.., Rhee P.L.., Koh K.C.., Paik S.W.., Rhee J.C.., Choi K.W.., Kim C.S.., Choi M.S.., Ryu K.H.., Lee H.Y.., Heo J.S.., Noh J.H.2000. One-week triple therapy for Helicobacter pylori a prospective, randomized study. Korean J. Gastroenterol. 35:16–22.
Sung K.C.2009. Effect of Cinnamomi Cortex aqueous extracts on reflux esophagitis induced by ligation of the pylorus and forestomach in rats. Kyungpook National University.1–101.
Wouden E.., Thijs J.., Zwet A.., Sluiter W.., Kleibeuker J.1999. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a metaanalysis. Am. J. Gastroenterol. 94:1751–1759.
Yam M.F.., Ang L.F.., Salman I.M.., Ameer O.Z.., Lim V.., Ong L.M.., Ahmad M.., Asmawil M.Z.., Basir R.2009. Orthosiphon stamineus leaf extract protects against ethanol-induced gastropathy in rats. J. Med. Food. 12(5):1089–1097.

Zhu Y.P.1998. Chinese materia medica chemistry, pharmacology and applications. pp. 353-356, Harwood Academic Publshers, The Netherlands.
Go to : 
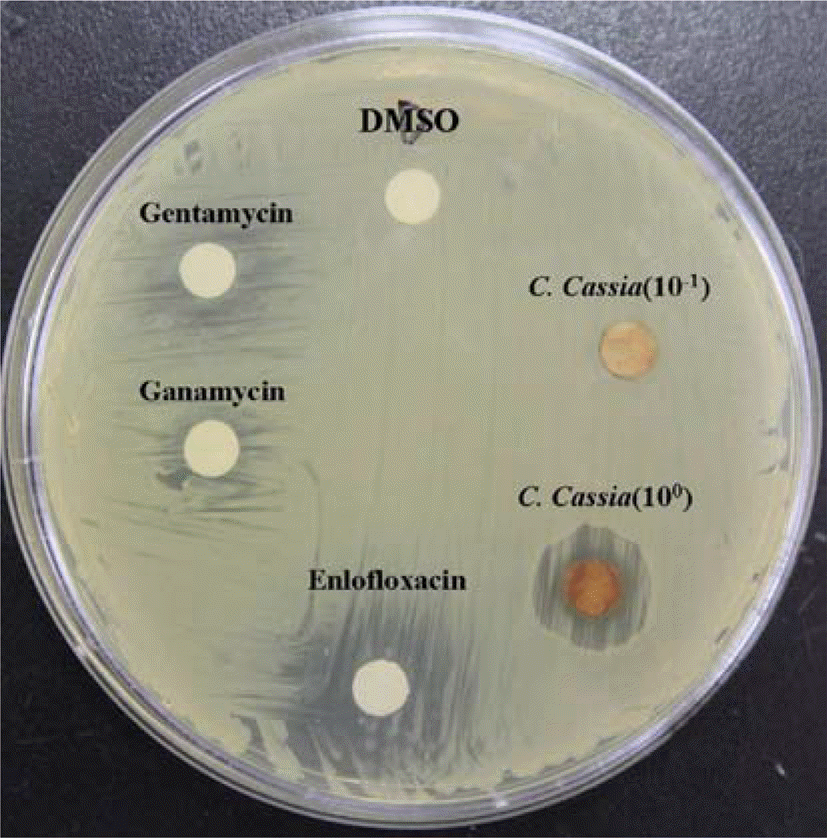 | Figure 1.The anti-H. pylori activities of Cinnamomum cassia extract using the paper disk diffusion bioassay. The diameter of clear zone reveals anti-H. pylori activities of the inoculated materiel. C. cassia (100): Cinnamomum cassia extract 1.5 mg, C. cassia (10−1): Cinnamomum cassia extract 0.15 mg. |
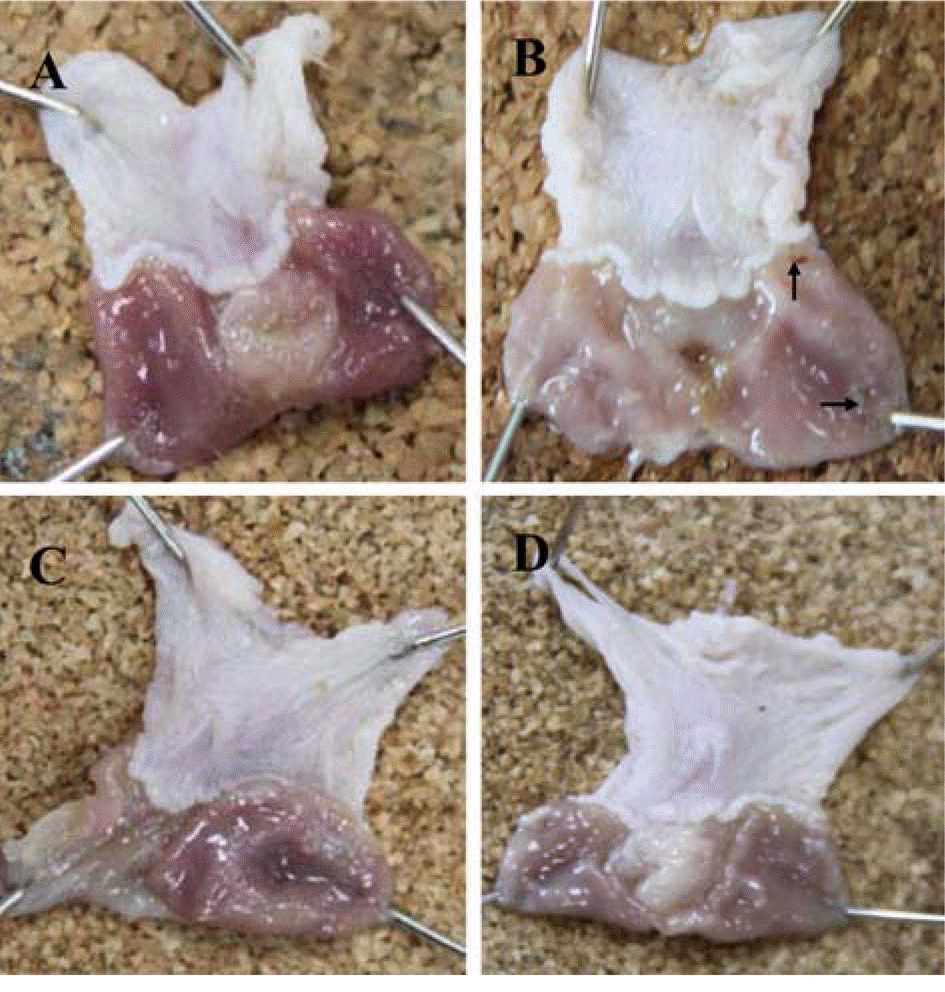 | Figure 2.Gross findings of the stomach in the study on therapeutic effects of H. pylori infection with Cinnamomum cassia extract. A: H. pylori+Cinnamomum cassia, B: H. pylori+ PBS (black arrow: ulcer lesions), C: PBS+Cinnamomum cassia, D: PBS+PBS. |
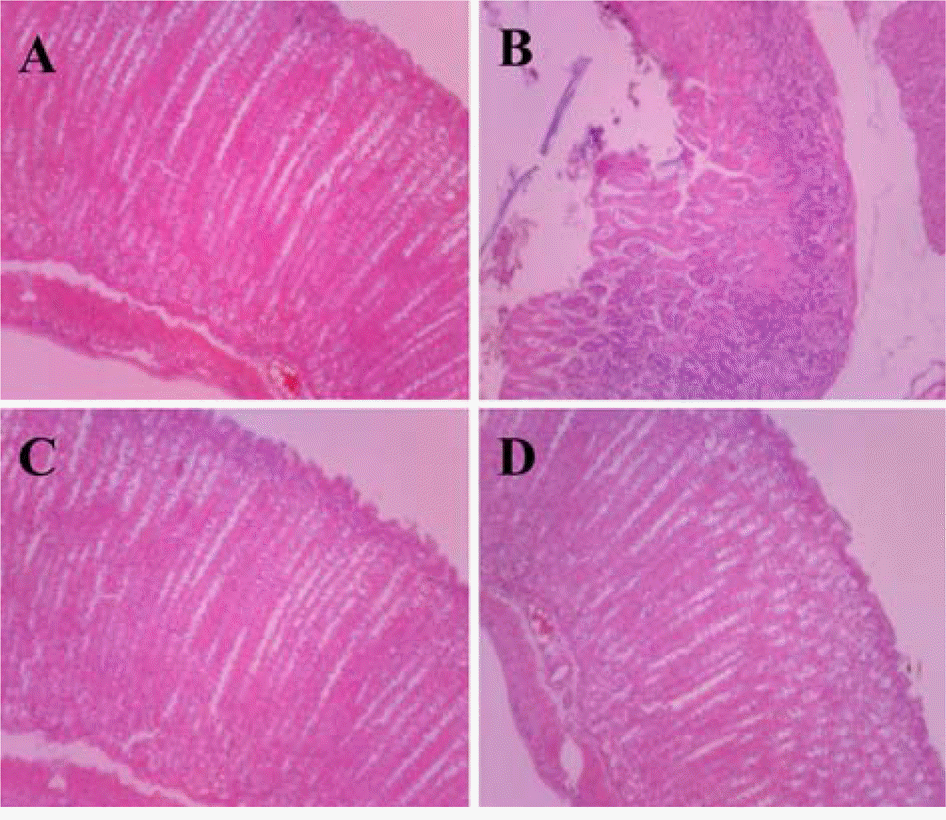 | Figure 3.Histopathological findings of the stomach in the study on therapeutic effects of H. pylori infection with Cinnamomum cassia extract. A: H. pylori+Cinnamomum cassia, B: H. pylori+ PBS (Cellular degeneration and atrophy in the mucous layer), C: PBS+Cinnamomum cassia, D: PBS+PBS. |
Table 1.
Anti-H. pylori activities of Cinnamomum cassia extract using the paper disk diffusion bioassay
Table 2.
Results of body weight changes of anti-H. pylori activities of Cinnamomum cassia extract using in vivo study
| Group | Treatment | Body weights (gram) | ||||
|---|---|---|---|---|---|---|
| O w.p.i.a | 1 w.p.i. | 2 w.p.i | 3 w.p.i | 4 w.p.i | ||
| I | H. pylori+Cinnamomumb | 135.5± 2.05 | 137.0± 2.55 | 141.0± 2.00∗ | 144.5± 3.55∗ | 146.0± 1.55∗ |
| II | H. pylori+PBS | 135.5±4.00 | 135.0± 3.00 | 136.0± 2.55 | 136.5± 2.55 | 137.0± 1.55 |
| III | PBS+Cinnamomum | 135.0± 5.10 | 141.0± 4.25 | 145.0± 4.00∗ | 149.5± 2.55∗ | 153.0± 2.40∗ |
| IV | PBS+PBS | 135.5± 4.15 | 139.0± 3.55 | 144.0± 3.15∗ | 148.0± 2.30∗ | 152.0± 2.00∗ |
Table 3.
Results of rapid urease test with gastric mucosal tissues after the study on therapeutic effects of H. pylori infection with Cinnamomum cassia extract
| Group | Inoculationa | N | Positive reaction (Positive percent) b | Therapeutic No. (Therapeutic percent) | |
|---|---|---|---|---|---|
| H. pylori | Treatment | ||||
| I | Yes | Cinnamomum | 10 |
0∗ (0 %, CI c 0-25.9) |
10∗ (100 %, CI 74.1-100) |
| II | Yes | PBS | 10 |
10 (100 %, CI 74.1-100) |
0 (0 %, CI 0-25.9) |
| III | No | Cinnamomum | 10 | 0∗ (0 %, CI 0-25.9) | - |
| IV | No | PBS | 10 | 0∗ (0 %, CI 0-25.9) | - |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download