Abstract
The purpose of this study was to compare the skin changes in female SKH-1 hairless mice between UVB irradiated photoaged group and endogenous aged group. The UVB irradiation and endogenous aged groups showed poor skin conditions when compared with normal (N) group in terms of the skin erythema, water content and TEWL (transepidermal water loss). For the changes in gross observation and replica image analysis on wrinkle of the skin tissue, UVB irradiation group showed thicker, wider and deeper wrinkles than the changes seen in N group, whereas endogenous aged group showed thinner, narrower and shallower wrinkles than that of UVB irradiation group. In histopathological findings, UVB irradiation group and endogenous aged group showed thickened epidermis, increased dermal inflammatory cells, decreased collagen and elastic fiber content, increased number of degranulated dermal/subcutaneous mast cells, and lower expression quantity of TGF-β in dermal layer when compared with N group, but to a lesser extent in aged group than the changes in UVB irradiation group. UVB irradiation group and endogenous aged group showed significantly higher xanthine oxidase activity, lower superoxide dismutase and catalase activities, and higher expression of MMP-3 mRNA in skin than N group. Therefore, aspectual comparison of the skin change in hairless mice between photoaged and endogenous aged groups showed different each other, and these results will be useful for skin aging research.
Go to : 
REFERENCES
Aebi H.1974. Catalase. In Methods of Enzymatic Analysis. pp. 673-684, Academic Press, New York.
Blanken R.., Vilsteren M.J.T.., Tupker R.A.., Coenraads P.J.1989. Effect of mineral oil and linoleic-acid-containing emulsions on the skin vapour loss of sodium-lauryl-sulphate-induced irritant skin reactions. Contact Dermatitis. 20(2):93–97.

Chung J.H.., Seo J.Y.., Choi H.R.., Lee M.K.., Youn C.S.., Rhie G.., Cho K.H.., Kim K.H.., Park K.C.., Eun H.C.2001. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Invest. Dermatol. 117(5):1218–1224.

Cole N.., Sou P.W.., Ngo A.., Tsang K.H.., Severino J.A.., Arun S.J.., Duke C.C.., Reeve V.E.2010. Topical 'Sydney' Propolis Protects against UV-Radiation-Induced Inflammation, Lipid Peroxidation and Immune Suppression in Mouse Skin. Int. Arch. Allergy Immunol. 152(2):87–97.

El-Domyati M.., Attia S.., Saleh F.., Brown D.., Birk D.E.., Gasparro F.., Ahmad H.., Uitto J.2002. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 11(5):398–405.
Fisher G.J.., Datta S.C.., Talwar H.S.., Wang Z.Q.., Varani J.., Kang S.., Voorhees J.J.1996. Molecular basis of sun-induced premature skin ageing and retinoid. Nature. 379:335–339.
Fisher G.J.., Kang S.., Varani J.., Bata-Csorgo Z.., Wan Y.., Datta S.., Voorhees J.J.2002. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 138:1462–1470.

Fitzpatrick R.E.., Rostan E.F.2003. Reversal of photodamage with topical growth factors: a pilot study. J. Cosmet. Laser Ther. 5:25–34.

Ghersetich I.., Lotti T.., Campanile G.., Grappone C.., Dini G.1994. Hyaluronic acid in cutaneous intrinsic aging. Int. J. Dermatol. 33(2):119–122.

Haratake A.., Uchida Y.., Schmuth M.., Tanno O.., Yasuda R.., Epstein J.H.., Elias P.M.., Holleran W. M.1997. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J. Invest. Dermatol. 108(5):769–775.

Inomata S.., Matsunaga Y.., Amano S.., Takada K.., Kobayashi K.., Tsunenaga M.., Nishiyama T.., Kohno Y.., Fukuda M.2003. Possible involvement of gelatinase in basement membrane damage and wrinkle formation in chronically. J. Invest. Dermatol. 120(1):128–134.
Jiang S.J.., Chu A.W.., Lu Z.F.., Pan M.H.., Che D.F.., Zhou X.J.2007. Ultraviolet B-induced alterations of the skinbarrier and epidermal calcium gradient. Exp. Dermatol. 16(12):985–992.
Kim S.H.., Nam G.W.., Kang B.Y.., Lee H.K.., Moon S.J.., Chang I.S.2005. The effect of kaempferol quercetin on hyaluronan-synthesis stimulation in human keratinocytes (HaCaT). Korean J. Food Sci. Nutr. 31:97–102.
Lee K.K.., Kim J.H.., Cho J.J.., Choi J.D.1999. Inhibitory effects of 150 plant extracts on elastase activity, and their anti-inflammatory effects. Int. J. Cosmet. Sci. 21(2):71–82.

Lowry O.H.., Rosebrough N.J.., Farr A.L.., Randall R.J.1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193(1):265–275.

Makrantonaki E.., Zouboulis C. C.2007. Characteristics and pathomechanisms of endogeniously aged skin. Dermatol. 214(4):352–360.
Martin J.P.., Dailey M.., Sugarman E.1987. Negative and positive assays of superoxide dismutase based on hematoxylin autooxidation. Arch. Biochem. Biophys. 225(2):329–336.
Martin P.., Hopkinson-Woolley J.., McCluskey J.1992. Growth factors and cutaneous wound repair. Prog. Growth Factor Res. 4(1):25–44.

Massague J.1998. TGF-β signal transduction. Ann. Rev. Biochem. 67:753–791.
Matsumura Y.., Ananthaswamy H.N.2004. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 195(3):298–308.

Oba A.., Edwards C.2006. Relationships between changes in mechanical properties of the skin, wrinkling, and destruction of dermal collagen fiber bundles caused by photoaging. Skin Res. Technol. 12(4):283–288.

Peltonen J.., Kahari L.., Jaakkola S.., Kahari V.M.., Varga J.., Uitto J.., Jimenez S. A.1990. Evaluation of transforming growth factorβ and type procollagen gene expression in fibrotic skin diseases by in situ hybridization. J. Invest. Dermatol. 94(3):365–371.
Quan T.., He T.., Kang S.., Voorhees J.J.., Fisher G.J.2002. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J. Invest. Dermatol. 119(2):499–506.
Rittie L.., Fisher G.J.2002. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 1(4):705–720.

Sander C.S.., Chang H.., Salzmann S.., Muller, C.S. Ekanayake-Mudiyanselage S.., Elsner P.., Thiele J.J.2002. Photoaging is associated with protein oxidation in human skin in vivo. J. Invest. Dermatol. 118:618–625.

Schmid P.., Kunz S.., Cerletti N.., Mcmaster G.., Cox D.1993. Injury induced expression of TGF-β1 mRNA is enhanced by exogenously applied TGF-βs. Biochem. Biophys. Res. Comm. 194(1):399–406.

Seite S.., Zucchi H.., Septier D.., Igondjo-Tchen S.., Senni K.., Godeau G.2006. Elastin changes during chronological and photo-ageing: the important role of lysozyme. J. Eur. Acad. Dermatol. Venereol. 20(8):980–987.

Seo J.Y.., Cho K.H.., Eun H.C.., Chung J.H.2001. Skin aging from phenotype to mechanism. Korean J. Invest. Dermatol. 8(4):187–194.
Stripe F.., Della C.E.1969. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase(type D) oxidase (type O). J. Biol. Chem. 244(14):3855–3863.
Sunderkötter C.., Kalden H.., Luger T.A.1997. Aging and the skin immune system. Arch. Dermatol. 133(10):1256–1262.

Trevithick J.R.., Xiong H.., Lee S.., Shum D.T.., Sanford S.E.., Karlik S.J.., Norley C.., Dilworth GR.1992. Topical tocopherol acetate reduces post-UVB, sunburn-associated erythema, edema, and skin sensitivity in hairless mice. Arch. Biochem. Biophys. 296(2):575–582.

Waller J.M.., Maibach H.I.2006. Age and skin structure and function, a quantitativeapproach (II): protein, glycosaminoglycan, water, and lipidcontent and structure. Skin Res. Technol. 12(3):145–154.
Wlaschek M.., Briviba K.., Stricklin G.P.., Sies H.., Scharffetter-Kochanek K.1995. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J. Invest. Dermatol. 104(2):194–198.

Yaar M.., Gilchrist B.A.1999. Aging of skin. Dermatology in general medicine. pp. 1697-1706, Mcgraw-Hill, New York.
Go to : 
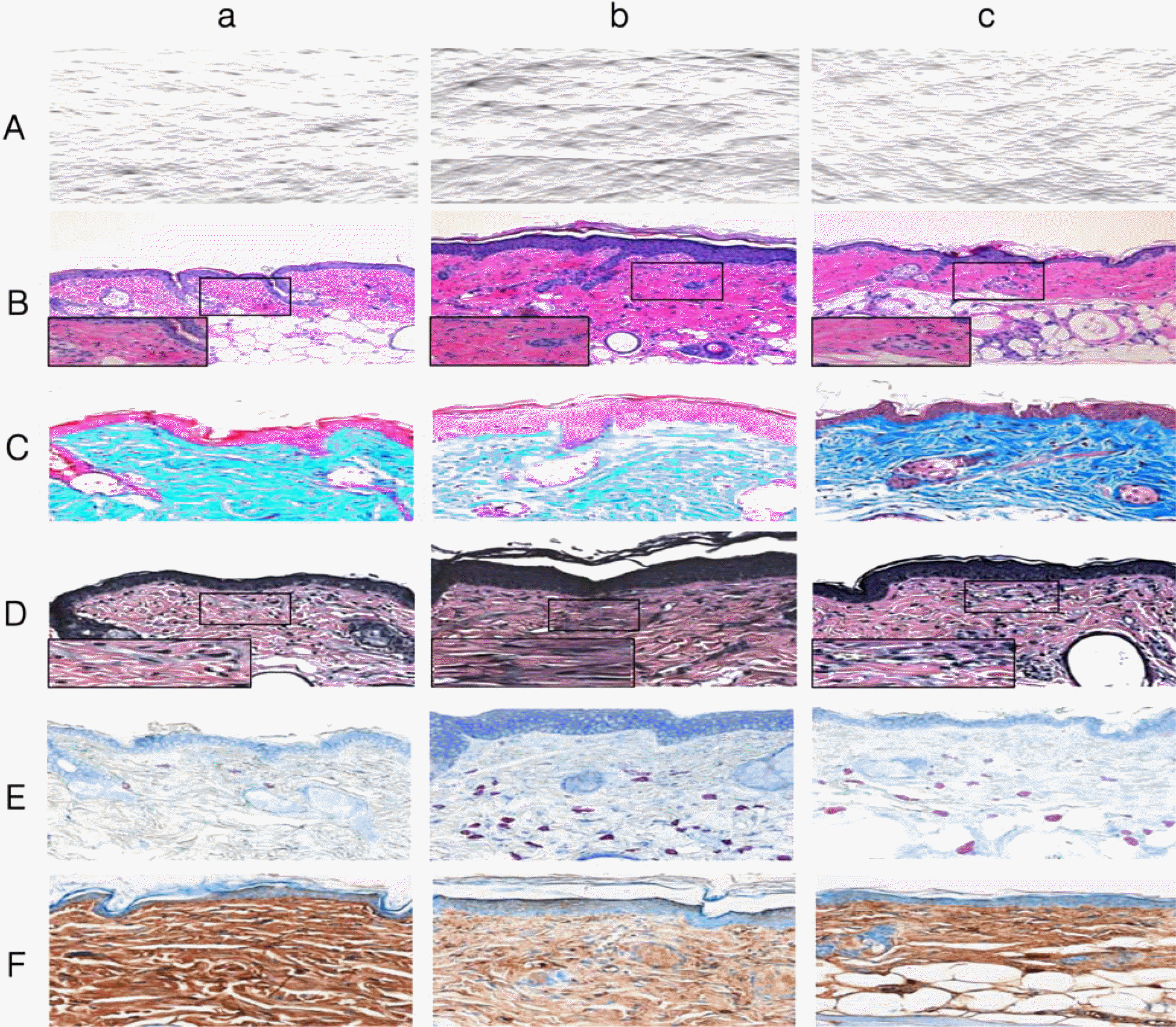 | Figure 1.Comparison of replica images in SKH-1 hairless mice at 6 weeks after the beginning of experiment a: normal group, b: UVB irradiation group, c: aged group (A). Histological observation on SKH-1 hairless mouse skin at 6 weeks after the beginning of experiment. H&E stain, ×100 & ×400 (enlarged box) (B). Masson's trichrome stain, ×200 (C). Verhoeff's stain, ×200 & ×400(enlarged box) (D). Toluidine blue stain, ×200 (E). Immunohistochemical staining for TGF-β in SKH-1 hairless mouse skin at 6 weeks after the beginning of experiment, ×200 (F). |
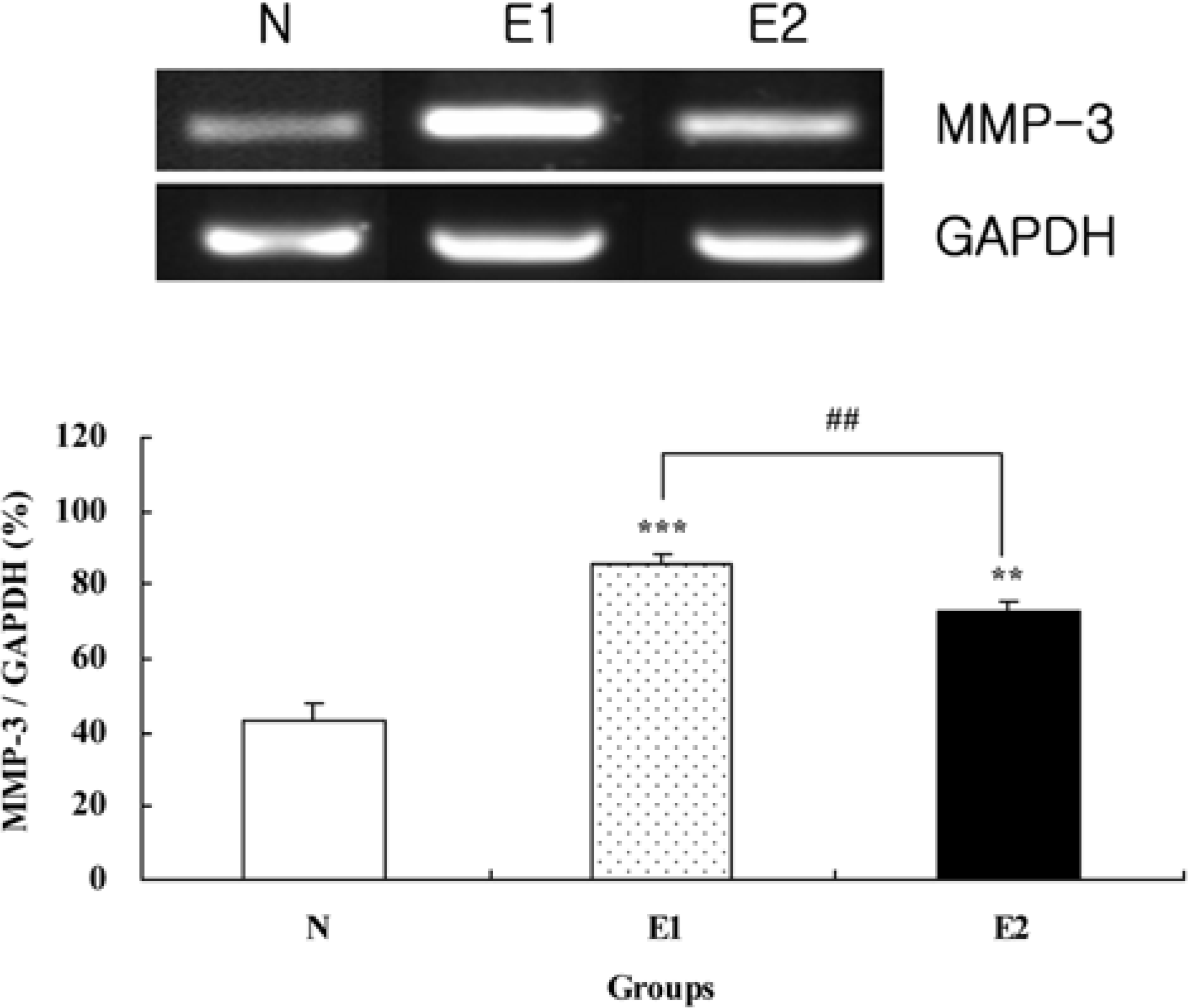 | Figure 2.Comparison of MMP-3 expression in hairless mice skin at 6 weeks after the beginning of experiment. Values are mean±SD of 6 mice. ∗∗P<0.01, ∗∗∗P<0.001 compared to the N group by ANOVA and Duncan's multiple range test. |
Table 1.
Nucleotide sequence of the primers and expected size of PCR products
| Items | Primers | Expected size (bp)3) | |
|---|---|---|---|
| GAPDH1) | Forward (5'→3') | CCCACTAACATCAAATGGGG | |
| Reverse (5'→3') | ACACATTGGGGGTAGGAACA | 478 | |
| MMP-32) | Forward (5'→3') | TAGCAGGTTATCCTAAAAGCA | |
| Reverse (5'→3') | CCAGCTATTGCTCTTCAAT | 317 |
Table 2.
Comparison of erythema index, water capacity, TEWL in SKH-1 hairless mice skin at 6 weeks after the beginning of experiment
| Items | Normal | Experimental | |
|---|---|---|---|
| N | E1 | E2 | |
| Erythema index1) | 98.07±20.83 | 261.67±68.85∗∗∗ | 208.81±31.01∗∗ |
| Water capacity1) | 61.83±6.90 | 43.40±10.87∗ | 49.42±6.19 |
| TEWL2) | 6.67±1.42 | 47.84±20.95∗∗∗ | 6.43±1.73### |
Table 3.
Comparison of wrinkle parameters in SKH-1 hairless mice skin at 6 weeks after the beginning of experiment
| Items | Normal | Experimental | |
|---|---|---|---|
| N | E1 | E2 | |
| Total wrinkle area (mm2) | 0.36±0.10 | 2.66±0.21∗∗∗ | 0.67±0.15∗∗### |
| No. of wrinkles | 18.80±3.27 | 66.00±4.42∗∗∗ 3 | 30.80±7.43∗∗### |
| Total length (mm) | 5.04±1.26 | 29.96±2.06∗∗∗ | 9.10±2.15∗∗### |
| Mean length (mm) | 0.27±0.02 | 0.46±0.03∗∗∗ | 0.30±0.02∗### |
| Mean depth (m) | 47.59±2.99 | 51.39±2.21∗∗∗ | 48.23±0.21∗## |
Table 4.
Comparison of xanthine oxidase (XO), superoxide dismutase (SOD) and catalase activities (CAT) in SKH-1 hairless mice skin at 6 weeks after the beginning of experiment
| ltems | Normal | Experimental | |
|---|---|---|---|
| N | E1 | E2 | |
| XO1) | 3.69±0.40 | 9.24±01.55∗∗∗ | 5.77±1.23∗## |
| SOD2) | 20.49±2.02 | 14.71±0.98∗ | 12.89±1.01∗∗ |
| CAT3) | 8.10±1.45 | 3.47±1.47∗∗ | 4.54±1.06∗∗ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download