Abstract
Multiple DNases were identified from Haemonchus contortus intestine based on previous studies. The DNases detected at 34, 36 and 38.5 kDa had diverse characteristics. Some of them had characteristics similar to those of mammalians and others had unusual characteristics. This study was carried out to fractionate worm intestinal DNases from other proteins using phenyl Sepharose chromatographic methods. All DNases detected from Haemonchus contortus intestine were fractionated in the flowthrough of phenyl Sepharose, indicating the worm DNases are hydrophilic. The DNases were enriched five-fold in the flowthrough fraction while additional steps are required for isolation of the worm DNases. Thus, fractionation with phenyl Sepharose could be used as a good initial step to enrich and separate DNases from other proteins.
Go to : 
REFERENCES
Barry M.A.., Eastman A.1993. Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch. Biochem. Biophys. 300(1):440–450.

Coles G.C.., Jackson F.., Pomroy W.E.., Prichard R.K.., von Samson-Himmelstjerna G.., Silvestre A.., Taylor M.A.., Vercruysse J.2006. ).The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136(3-4):167–185.

Enari M.., Sakahira H.., Yokoyama H.., Okawa K.., Iwamatsu A.., Nagata S.1998. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 391(6662):43–50.

Hedgecock E.M.., Sulston J.E.., Thomson J.N.1983. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 220(4603):1277–1279.
Jasmer D.P.., McGuire T.C.1991. Protective immunity to a blood-feeding nematode (Haemonchus contortus) induced by parasite gut antigens. Infect. Immun. 59(12):4412–4417.

Jasmer D.P.., Yao C.., Rehman A.., Johnson S.2000. Multiple lethal effects induced by a benzimidazole anthelmintic in the anterior intestine of the nematode Haemonchus contortus. Mol. Biochem. Parasitol. 105(1):81–90.

Karanu F.N.., Rurangirwa F.R.., McGuire T.C.., Jasmer D.P.1993. Haemonchus contortus: identification of proteases with diverse characteristics in adult worm excretory-secretory products. Exp. Parasitol. 77(3):362–371.

Kwak D.., Jasmer D.P.2003. ).Non-classic characteristics define prominent DNase activities from the intestine and other tissues of Haemonchus contortus. Exp. Parasitol. 104(3-4):131–139.

Kwak D.., Jasmer D.P.2004. ).Intestinal DNases of 36 and 38.5kDa from the parasitic nematode Haemonchus contortus have non-classic DNase characteristics and produce DNA fragments with 3'-hydroxyls. Exp. Parasitol. 108(3-4):142–153.

Lacey E.1988. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int. J. Parasitol. 18(7):885–936.

Lubega G.W.., Prichard R.K.1990. Specific interaction of benzimidazole anthelmintics with tubulin: high-affinity binding and benzimidazole resistance in Haemonchus contortus. Mol. Biochem. Parasitol. 38(2):221–232.

Mak C.H.., Ko R.C.1999. Characterization of endonuclease activity from excretory/secretory products of a parasitic nematode, Trichinella spiralis. Eur. J. Biochem. 260(2):477–481.

Parrish J.., Li L.., Klotz K.., Ledwich D.., Wang X.., Xue D.2001. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature. 412(6842):90–94.
Parrish J.Z.., Xue D.2003. Functional genomic analysis of apoptotic DNA degradation in C. elegans. Mol. Cell. 11(4):987–996.
Ribeiro J.M.., Carson D.A.1993. Ca2+/Mg(2+)-dependent endonuclease from human spleen: purification, properties, and role in apoptosis. Biochemistry. 32(35):9129–9136.
Rodriguez A.M.., Rodin D.., Nomura H.., Morton C.C.., Weremowicz S.., Schneider M.C.1997. Identification, localization, and expression of two novel human genes similar to deoxyribonuclease I. Genomics. 42(3):507–513.

Saraste A.., Pulkki K.2000. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 45(3):528–537.

Shiokawa D.., Tanuma S.1998. ).Molecular cloning and expression of a cDNA encoding an apoptotic endonuclease DNase gamma. Biochem. J. 332(Pt 3):713–720.
Shiokawa D.., Tanuma S.1999. DLAD, a novel mammalian divalent cation-independent endonuclease with homology to DNase II. Nucleic Acids Res. 27(20):4083–4089.

Shiokawa D.., Tanuma S.2001. Characterization of human DNase I family endonucleases and activation of DNase gamma during apoptosis. Biochemistry. 40(1):143–152.
Go to : 
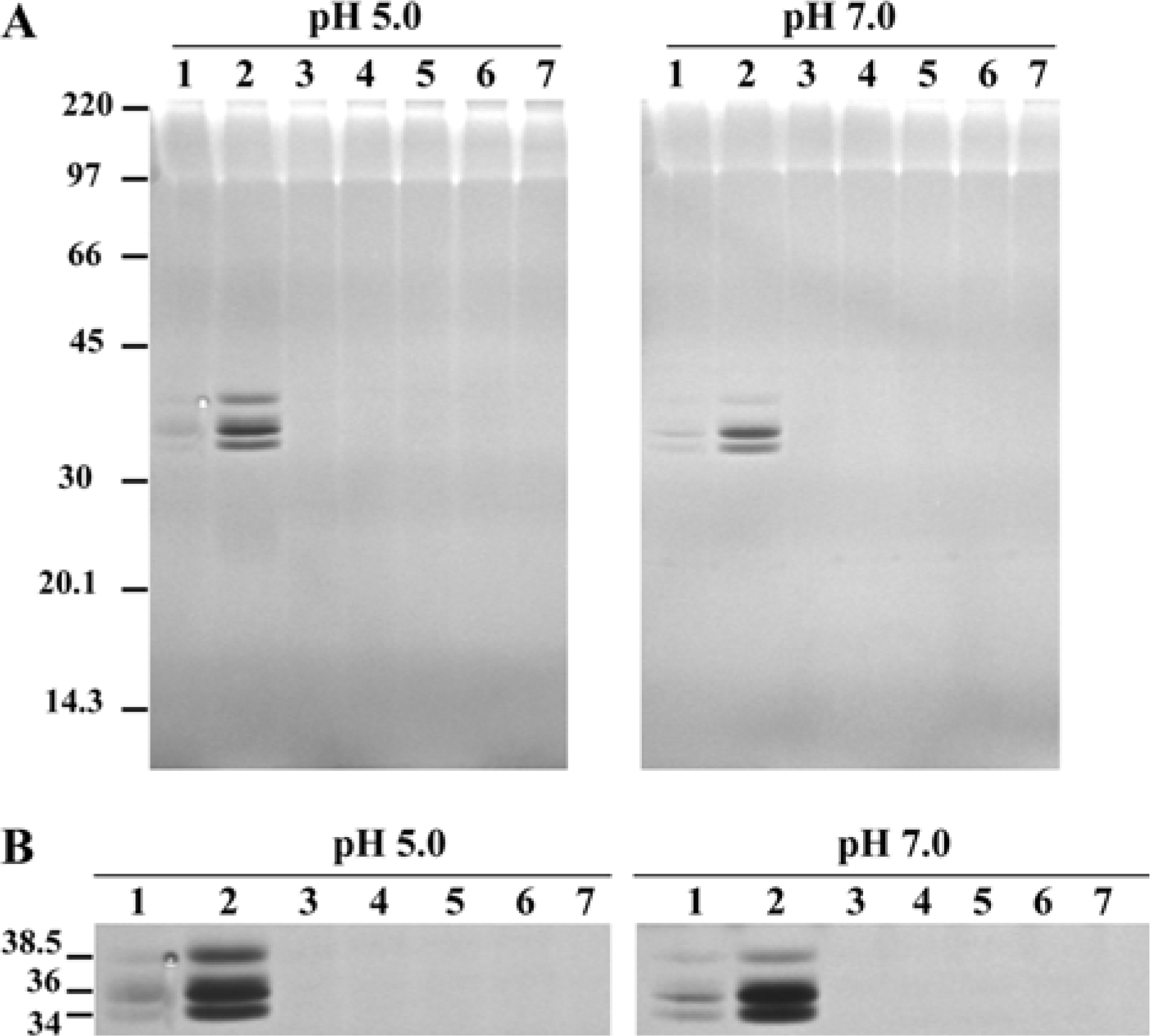 | Figure 1.DNase activities from the intestine of Haemonchus contortus adult worms. (A) Zymograms of DNases from the intestine of H. contortus in phenyl Sepharose fractions. Samples (100 µg of protein), including original intestinal lysates (lane 1), flowthrough (lane 2), 0.5 M (lane 3), 0.3 M (lane 4), 0.2 M (lane 5), 0.1 M (lane 6) or 0.0 M (lane 7) of (NH4)2SO4 fractions, were separated by SDS-PAGE (10% gel containing 200 µg/mL salmon sperm DNA) and incubated in buffers (pH 5.0 or 7.0) containing 2 mM CaCl2/MgCl2 for 88 h. Mrs of DNases were identified by staining the gel with ethidium bromide. Molecular markers are indicated in kDa on the left. (B) Selected enlargement and longer incubation (136 h) of panel A to show individual DNases better. Mrs of DNases are indicated in kDa on the left. |
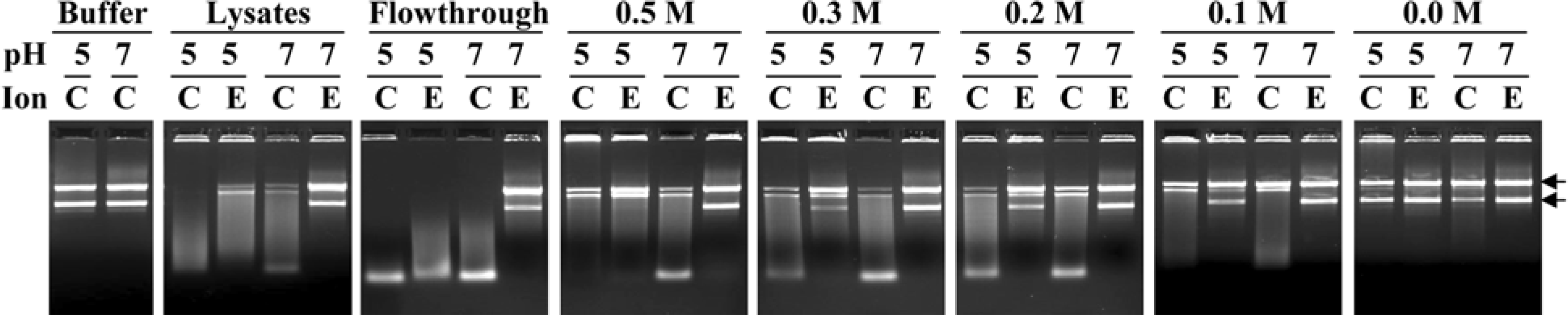 | Figure 2.Plasmid digestion assays for DNase activities in phenyl Sepharose fractions of Haemonchus contortus intestine. Samples (400 ng protein), including unfractionated intestinal lysates, flowthrough, 0.5, 0.3, 0.2, 0.1 or 0.0 M (NH4)2SO4 fractions of phenyl Sepharose, were incubated with plasmid DNA (400 ng) at 37oC for 8 h in buffers (pH 5.0 or 7.0) with 2 mM CaCl2/MgCl2 (C) or 10 mM EDTA (E). Buffers without sample were incubated for 8 h as a control. The DNA fragments produced were analyzed by staining agarose gel (0.8%) with ethidium bromide. Arrows on the right refer to estimated sizes of two forms of uncut plasmid DNA at 4 kb (upper) and 2.5 kb (lower), respectively. |
 | Figure 3.3'-End labeling of plasmid DNA fragments produced by DNase activities from unfractionated lysates and phenyl Sepharose flowthrough fraction of Haemonchus contortus intestine. Digested plasmid DNA (see Figure 2) was treated with (+) or without (-) alkaline phosphatease (ALP) at 37oC for 1 h, and then subjected to 3'-end labeling reactions. DNA (20 ng) separated on agarose gel (0.8%) was transferred to nylon membranes. Labeled ends were detected by an enhanced chemiluminescence detection system. Buffer without sample was evaluated as a control. The ratio of signal obtained with (+) or without (-) ALP was determined with densitometric quantitation. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download