Abstract
Purpose
Guidelines need to be tailored to where they are applied. We aimed to describe the distinctive asthma severity profile and the pattern of controller prescription in Korean children.
Methods
Twelve pediatric allergists from tertiary medical centers reviewed medical records of all asthmatic children who visited their clinics between September 1 and November 30 of 2013. Controller prescriptions were re-classified into 4 categories, then the prevalence of each asthma severity category and the controller prescription patterns according to asthma severity assessed by a Western (Global Initiative for Asthma, GINA) and an Asia-Pacific (Japanese Pediatric GuideLine, JPGL) guideline were evaluated.
Results
A total of 840 cases were reviewed. Both GINA and JPGL revealed that 328 (39.0%) and 249 (29.6%) subjects had intermittent asthma whereas 24 (2.9%) and 21 (2.5%) subjects had severe persistent asthma, respectively. Although higher category controllers tended to be prescribed to those who had more severe asthma, there was much overlap in categories of prescribed controllers between groups with regard to asthma severities. Leukotriene receptor antagonists (LTRA) was the most frequently prescribed as a single controller (40%) or as an add-on medication (19%) in the group of asthmatic children <6 years.
Conclusions
Korean children have distinctive patterns of asthma severity and management strategies with a lower prevalence of severe asthma and a preference toward LTRA rather than low dose inhaled corticosteroids (ICS) alone or add-on long-acting beta-agonist (LABA) in the group of <6 year-old asthmatics that has not been predicted in Western countries. Thus, strategies tailored to regional situations need to be developed and recommended.
Guideline-based management of asthma is highly recommended today as it enables physicians to achieve and maintain control of the disease with minimal side effects caused by medications.1 In this regard, the assessment of asthma severity is fundamental as it can help physicians determine the most appropriate initial medications as well as the frequency and methods of monitoring symptoms.2 Even it enables us to predict the outcome of childhood asthma.3 Yet, as guidelines or recommendations are developed by validating the safety, efficacy, and cost effectiveness of their own available resources, guidelines recommend differently according to where they would be applied to.4
Asian countries have shown distinctive characteristics of asthma compared to those of Western countries.56 For example, the Japanese population, which resides closest to Korea in the Asia-Pacific region, has a high prevalence of asthma but a low prevalence of severe asthma. Thus, Japanese Pediatric GuideLine (JPGL)7 recommends their own strategy which differs much from that of Global Initiative for Asthma (GINA)8 or National Asthma Education and Prevention Program (NAEPP).9
In Korea, translated guidelines of GINA8 and NAEPP9 have long been adopted for use to manage asthmatic children. However, a considerable number of patients, owing to cultural taboos, are still assumed to have steroid phobia and often prefer oral medication.1011 Thus, in order to gather data on the current status of asthma prevalence, severity, and the pattern of management in the real practice setting, we conducted a cross-institutional survey over 12 pediatric allergy centers at different tertiary hospitals. We aimed to describe the profile of asthma-severity and their prescription pattern of controllers using one of the existing Western (GINA)8 and Asia-Pacific (JPGL)7 guidelines as a reference.
The present study was designed as a cross-institutional survey to evaluate the distribution of asthma severity and the pattern of controller prescriptions in Korean asthmatic children. Pediatric allergists in 12 separate tertiary hospitals participated in the present study. The authors comprehensively reviewed the medical records of asthmatic children between September 1 and November 30 of 2013, the most recent 3 months before the survey. The institutional review boards of each hospital approved the study protocol.
All asthmatic children, regardless of their asthma duration, who visited the outpatient asthma clinic during the study period, were investigated.
The diagnosis of asthma was verified according to age-specific asthma criteria. For those <3 years of age, a positive modified asthma predictive index (mAPI) was used to confirm the asthma.12 For those ≥6 years of age, typical asthmatic symptoms (wheezing, dyspnea, severe cough and/or exercise intolerance) with objective measures of asthmatic airways, i.e. bronchial reactivity assessed by either the methacholine PC20 of <16 mg/mL or by the mannitol PD15 of <635 mg, reversibility verified by the change in forced expiratory volume in 1 second exceeds 12 percent of baseline, variability in peak expiratory flow (PEF), and/or elevated exhaled nitric oxide (eNO) levels, were used.8 For each institution, examiners performed spirometry and bronchodilator testing according to the ATS/ERS guideline.13 To assess reversible bronchial obstruction, subjects inhaled a total of 200 mcg of albuterol and repeated spirometry with an interval of 10-15 minutes. To evaluate variability in their PEF, subjects were asked to perform peak flowmetry at home twice a day over 2 weeks. Asthma was labelled when the average of the difference between the maximum and the minimum values for the day, expressed as a percentage of the mean daily PEF, exceeds 13%.14 The level of eNO was measured according to the manufacturer's instructions. An elevated eNO >35 ppb,15 which is a significantly higher value than that of 95% upper limit of Korean healthy elementary school children,16 was interpreted as having asthma. However, low eNO could not exclude asthma because eNO can vary according to the age, height, weight, gender, the presence of atopy, and the analytic instruments.17 Finally, for those who were between 3 and 5 years of age, typical asthmatic symptoms plus either a positive mAPI or objective measures of asthmatic airways, i.e. methacholine end-point concentrations of <8 mg/mL, were used to confirm the diagnosis of asthma.
If subjects had respiratory or cardiac diseases which were not discernible from bronchial asthma, they were excluded from the analysis.
The medical records of the children's asthma-controlled status were comprehensively reviewed, including patients' reply on daytime symptoms, nighttime symptoms, frequency of bronchodilator use, and limitations in daily activities during the month prior to their visit. In addition, the results of their pulmonary function tests, bronchial reactivity, bronchial reversibility, peak expiratory flow variability, and exhaled nitric oxide were reviewed, if available. For the other allergic diseases accompanying the asthma, i.e. atopic dermatitis, allergic rhinitis and allergic conjunctivitis, diagnosis was made clinically, based on the answers to a brief question on their existence, by the allergists themselves who recorded the medical chart.
For each subject, asthma severity was assessed based on the medical records according to both criteria of GINA and JPGL, respectively.78 According to the GINA criteria, for those who were newly diagnosed or controller-naïve, symptoms occurring less than once a week and nocturnal symptoms not more than twice a month were classified as indicating mild-intermittent severity.8 Symptoms occurring more than once a week or nocturnal symptoms more than twice a month were classified as indicating mild-persistent severity. Daily symptoms and/or nocturnal symptoms occurring more than once a week were classified as indicating moderate-persistent severity. Finally, Symptoms occurring daily with frequent nocturnal symptoms or exacerbations were classified as indicating severe-persistent severity.
As for the JPGL criteria, we evaluated the severity of asthma exacerbation based on the presence and the degree of breathless wheezing, retraction, prolonged expiration, posture, cyanosis, and respiratory rate.7 If subjects had symptoms less than several times a year with brief instances of exacerbation, we regarded them as having intermittent asthma. Symptoms occurring less than once a week with the subjects able to lead a normal daily life were classified as indicating mild-persistent severity. Symptoms which occurred more than once a week with instances of moderate or severe attacks were classified as indicating moderate-persistent severity. Finally, those who suffered symptoms daily, with moderate or severe attacks occurring more than several times a week, and trouble leading a normal daily life were classified as having severe persistent asthma.7
If subjects achieved complete control while taking controller medications,8 we reviewed their medical records on the prescriptions and the level of control up to 1 year. Their asthma severity was determined by the lowest level of treatment required to achieve patient's best level of asthma control.18
To describe the prescription patterns of asthma controller medications, the authors re-classified controller medications recommended at either guideline into 4 categories:78 Category 1, no controller medication with occasional short-acting beta-agonists (SABA); Category 2, single controllers, i.e. low dose inhaled-corticosteroids (ICS), leukotriene receptor antagonists (LTRA), or theophylline alone; Category 3, up-dosing or two-drug combination, i.e. medium- or high-dose ICS alone, ICS+ long-acting beta-agonists (LABA), or ICS+ LTRA; and Category 4, ICS+ LABA+ LTRA, or oral-corticosteroids (OCS).
Only cases containing all the information on diagnosis, the level of control and the controller prescription were gathered from each pediatric allergist. No further case was excluded during analyses thereafter.
Only descriptive measures were used in the present analysis. If the subjects visited the allergy clinics several times during the period, the highest levels of severity were labelled to their own asthma severity.
A total of 840 children were included in this analysis and their demographic and clinical characteristics are listed in Table 1. More than half of the subjects were at least 6 years old (448/840, 53.3%), were boys (554/840, 67.0%), had a familial history of allergic rhinitis (435/840, 51.8%) and were sensitized to aeroallergens (466/840, 51.4%). Among the 670 children aged 3 years or older, the diagnosis of asthma was made through presenting bronchial hyperresponsiveness in 318 (47.5%) subjects. Since only few (24/222, 10.8%) subjects aged between 3 to 5 years could perform the lung function test, the subjects (198/222, 89.2%) of that age group were diagnosed to have asthma clinically, based on the typical asthmatic symptoms plus positive mAPI.
The distribution of clues associated with the level of control is depicted in Fig. 1. A total of 672 of 840 (80.0%) children presented with daytime symptoms occurring less than once a week, while 22 (2.6%) children suffered almost daily. Among 840 children, 726 (86.5%) complained of nighttime symptoms occurring less than twice per week, while 10 (1.2%) children suffered almost daily. Five hundred and two children (59.8%) out of 840 did not use rescue medicine and 716 (85.2%) subjects had no limitation of activities during most of the study period.
Asthma severity categorized according to GINA and JPGL of the total subjects are described in Fig. 2. According to the GINA criteria, 328 of 840 (39.0%) cases were mild-intermittent, 327 (38.9%) cases were mild-persistent, 161 (19.2%) cases were moderate-persistent and the other 24 (2.9%) cases were severe-persistent. According to the JPGL criteria, 249 of 840 (29.6%) cases were intermittent, 314 (37.4%) cases were mild-persistent, 256 (30.5%) cases were moderate-persistent and the other 21 (2.5%) cases were severe-persistent. In 625 of 840 (74.4%) cases, severity assessed by both criteria was well-matched, whereas the other 215 (25.6%) cases presented discrepancy between severities classified by both criteria. Eighty-one of 840 (9.6%) cases were classified as intermittent using GINA, but as persistent with JPGL criteria, and 2 (0.2%) cases were vice versa. In addition, while 108 of 840 (12.9%) cases were regarded as mild using GINA but moderate with JPGL criteria, 16 (1.9%) cases were regarded as mild using JPGL but moderate or severe with GINA criteria. Furthermore, 7 cases with severe asthma assessed using GINA and 4 cases with severe asthma using JPGL criteria were not considered severe when they were classified using the other criteria, respectively.
The patterns of controller prescriptions in all asthmatic children are listed in Table 2. LTRA alone was the most frequently prescribed medication. In as many as 179 (21.3%) out of 840, which is equal to the sum of the prescribed low- and medium-doses of ICS alone (160 and 19 subjects, each). In addition, the fixed dose combination of ICS and LABA was prescribed in 91 (10.8%). LABA and theophylline was not prescribed alone but in combination with other controller medications. In 425 (50.6%), ICS was prescribed alone or in combination with other controller medications, as was LTRA in 334 (39.8%) cases. Finally, no controller medications were prescribed in 196 of 840 (23.2%) cases whereas OCS was prescribed in as many as 40 (4.8%) cases.
The patterns of controller prescriptions according to asthma severity assessed by GINA are depicted in Fig. 3A. There was much overlap in categories of prescribed controllers between the groups with regard to asthma severities. Whereas only 95 of 328 (29%) children with mild-intermittent asthma were prescribed SABA as needed basis without any controller medication (Category 1), 190 (58%) and 31 (10%) of them were recommended to use Category 2 and Category 3 controller medications, respectively. For children with persistent asthma, the category numbers of controller medications that are most frequently prescribed at each asthma severity tended to increase along with asthma severity (in mild-persistent asthma, 208/327 [64%] at Category 2; in moderate-persistent asthma, 112/161 [69%] at Category 3; and in severe-persistent asthma, 23/24 [96%] at Category 4). Across all the asthma severity classes, LTRA was so widely prescribed that within the Category 2 medications the prescription rate of LTRA exceeded the rate of low dose ICS inhalation alone.
The patterns of controller medications prescribed according to JPGL asthma severity were similar to those prescribed according to GINA guidelines. While controller medications were prescribed to 145 of 249 (59%) children with intermittent asthma (Category 2 in 137 [55%] and Category 3 in 8 [4%], respectively), no controllers were prescribed to only 93 (37%) children (Fig. 3B). For children with persistent asthma, the most frequent prescription category of each severity class tended to increase along with the increase in asthma severity (in mild-persistent asthma, 245/314 [78%] at Category 2; in moderate-persistent asthma, 138/256 [55%] at Category 3; and in severe-persistent asthma, 17/21 [81%] at Category 4). A high prescription rate of LTRA was still noted, especially in children with intermittent- or mild-persistent asthma; LTRA was prescribed in 99/249 (40%) of children with intermittent asthma (alone in 95 [38%], in combination with ICS in 4 [2%]) and in 171/314 (54%) of mild-persistent asthma (alone in 132 [42%], in combination with ICS in 36 [11%], and in combination with fixed dose ICS plus LABA in 3 [1%]).
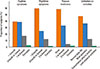
The prescription patterns of the different age groups are shown in Fig. 4. For those under the age of 6, LTRA was preferentially prescribed as a single controller medication to the low dose ICS (40% vs 29%), and also as an add-on medication to ICS+ LABA (19% vs 3%). For those 6 years or older, whereas LTRA and low dose ICS were similarly prescribed (21% vs 18%), ICS+LABA was preferred to the add-on LTRA (21% vs 7%).
This study demonstrated the current severity profile and the real-life practice characteristics in asthmatic children who visited allergy clinic in 12 tertiary hospitals in Korea. The proportion of severe asthma was as low as 2.9% (by GINA) or 2.5% (by JPGL), and two thirds or more of 840 subjects in this study had mild asthma. One fourth of subjects presented with discordant asthma severity assessments between the GINA and JPGL classification systems. Moreover, the prescription pattern did not match GINA, the world-widely accepted guideline: there was much overlap in categories of prescribed controllers between the groups concerning asthma severities. Although the most frequently prescribed controller in persistent asthma was ICS, LTRA was the preferred controller-of-choice among children under 6 years of age. This study is the first and the largest one describing the real-life practice characteristics of Korean asthmatic children and advocates the need for guidelines reflecting the current practice pattern in Korea.
Although previous population surveys on the asthma prevalence in Korean children showed that 8.3% to 10.3% of school-aged children19 and 13.3% to 15.4% of preschool ones had bronchial asthma,2021 no study was conducted with regard to the severity profile in Korean asthmatic children within the past 10 years. In 2000, population surveys were conducted in Korea as a part of the Asthma Insights and Reality in Asia Pacific (AIRIAP) program and were later reported as parts of tables or figures in previous reports.622 In the AIRIAP report, 884/3,207 (27.6%) subjects were children presenting with mild-intermittent severity in 60.1%, mild-persistent severity in 19.1%, moderate-persistent severity in 12.8%, and severe-persistent severity in 8.0% when asthma severity based on the symptom severity index was assessed.6 In another report on the same study, in addition, 401 (12.5%) subjects were Korean and they presented with mild-intermittent severity in 48%, mild-persistent severity in 19%, moderate-persistent severity in 19%, and severe-persistent severity in 14%.22 In the second phase of the AIRIAP report performed in 2006, 400/4,805 (8.3%) subjects were Koreans and their asthma severity were presented as mild-intermittent in 67%, mild-persistent in 22%, moderate-persistent in 9%, and severe-persistent in 2%.23 However, these data were not directly comparable to the results of the present study as our results were derived from subjects who visited allergy clinics by patients themselves.
In the present study, we described the distribution of asthma severity in Korean children using 2 distinctive criteria.78 Both criteria consistently revealed that mild asthma was frequently found in 67.0% (by JPGL) to 77.9% (by GINA) of subjects. On the other hand, severe asthma comprised <3% which is lower than that previously reported in Western countries.52425 The severity distribution towards a milder one is consistent with AIRIAP report that Korean subjects had milder diseases than others in the Asia-pacific region.23 This milder severity does not seem to be associated with the study periods or the participating centers. In the designing step, we confined the study period from September 1 through November 30 both to minimize seasonal variation in asthma exacerbation,2627 which is notorious for asthma exacerbation in Korea.28 The huge daily temperature difference, higher dust mite concentration, and the commonly observed outbreak of respiratory infections can easily exacerbate asthma.262728 Moreover, that period does not include vacation periods during which school-aged, chronic, stable asthmatics undergo their regular follow-ups. In addition, we gathered information from children who visited our clinics mainly in tertiary hospitals, which may have affected the outcome toward rather severe ones by excluding those who initially had mild-intermittent asthma or those who were former, but not current, asthmatics. Although the medical chart review tends to assess asthma severity as milder one than the direct assessment does,29 the lower prevalence of severe asthma may be associated with the distinct Korean national insurance system. Since it covers all residents with low medical costs30 the barrier to assess asthma care and medication is not so high compared to that of foreign countries, which may augment the compliance to controller medication and decrease the event of exacerbation.
Various controller medications are prescribed. We observed a wide discrepancy between the observed asthma severity and the patterns of prescriptions. This discrepancy was not confined to those whose severities were differently classified by the 2 guidelines but expanded to almost all groups of subjects. Even the 12 pediatric allergy specialists who were fully aware of guidelines and the severity assessment, prescribed controllers did not match the current guidelines, which may imply that there exists some acquaintance that each physician might have believed that another medication is more suitable than the guideline-based one to control subjects' symptoms. Moreover, we were able to observe several characteristic patterns: single drugs tended to be prescribed to those who had milder asthma, whereas combination drugs tended to be prescribed to those who had more severe asthma; LTRA was so widely prescribed that it might exceeded the low dose ICS as a monotherapy. It also exceeded the fixed combination with LABA as an add-on medication to initial low dose ICS, especially in subjects less than 6 years of age. The higher LTRA prescription rate may have been affected by the steroid phobia among parents of asthmatic children or by the belief that there would be greater adherence to an oral controller than to inhaled controller medications.31 On the other hand, this discrepancy may also be, in part, associated with recent changes in asthma management. Especially in mild persistent asthma, subjects who adopted the use of steroids as maintenance and reliever therapy (SMART)3233 or the short-course of double-dose ICS with no controllers34 may have counted as prescribing higher category medications, which could contribute to the higher rate of the discrepancy.
There are limitations in our study. First, we selected a retrospective design. For those already using controllers, extensive review on their previous medical records was inevitable to accurately determine the minimum level of treatment to maintain the well-controlled asthma. Second, since subjects enrolled in this study could not cover all asthmatic children geographically, some may doubt they may not represent Korean asthmatic children. Nonetheless, this cross-institutional study has its own value since no previous report has presented available data, not to speak of one assessed by the qualified pediatric allergy specialists as did in this study, on the Korean children in this regard. Third, it is hard to discern severe asthma from uncontrolled flare-ups.35 If we had gathered more information on the compliance and competence to medications as well as the control of comorbid diseases, which the most recent GINA guidelines emphasizes,14 it might have been easier to differentiate both conditions. For this reason, further replication studies are warranted to generalize the milder asthma severity in Korean children. On the other hand this study has strengths worth mentioning. Pediatric allergists themselves took histories related to asthma severity. In addition, we described the characteristics of asthma severity multidimensionally by contrasting distributions classified by a Western (GINA)- and an Asia-pacific (JPGL)- guidelines at the same time.
In summary, the distinctive patterns of asthma severity distribution were demonstrated in Korean children, with the low prevalence of severe asthma. Moreover, the patterns of prescriptions did not match the recommended medication groups according to the severity assessed by either GINA or JPGL, with the higher prescription rate of LTRA in a group of children less than 6 years. These results advocate that the guideline should be tailored to where it is applied.
Figures and Tables
Fig. 2
Distribution of asthma severity in 840 children as categorized by the 2 distinctive asthma guidelines, GINA and JPGL. GINA, Global Initiative for Asthma; JPGL, Japanese Pediatric Guideline.

Fig. 3
Prescription patterns of controller medications according to asthma severity assessed using (A) GINA and (B) JPGL. Shaded areas stand for the proportion of LTRA-containing modalities. GINA, Global Initiative for Asthma; ICS, inhaled corticosteroid; JPGL, Japanese Pediatric Guideline; LABA, long-acting beta-2 receptor agonist; LTRA, leukotriene receptor antagonist; SABA, short-acting beta-2 receptor agonist.

Fig. 4
Prescription patterns of controller medications among different age groups. Shaded areas stand for the proportion of LTRA-containing modalities. GINA, Global Initiative for Asthma; ICS, inhaled corticosteroid; JPGL, Japanese Pediatric Guideline; LABA, long-acting beta-2 receptor agonist; LTRA, leukotriene receptor antagonist; SABA, short-acting beta-2 receptor agonist.
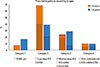
Table 1
Characteristics of the asthmatic children in this study

Table 2
Patterns of controller prescriptions applied to the 840 asthmatic children

ACKNOWLEDGMENTS
The Asthma Study Group for Preschool Children is supported by the Korean Academy of Pediatric Allergy and Respiratory Diseases (KAPARD). All of the authors of this study are members of KAPARD.
References
1. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004; 170:836–844.
2. National Asthma Education and Prevention Program (US). Expert panel report 2: guidelines for the diagnosis and management of asthma 1997. Bethesda (MD): National Institutes of Health, National Heart, Lung, and Blood Institute;1997.
3. de Marco R, Marcon A, Jarvis D, Accordini S, Almar E, Bugiani M, et al. Prognostic factors of asthma severity: a 9-year international prospective cohort study. J Allergy Clin Immunol. 2006; 117:1249–1256.
4. Myers TR. Guidelines for asthma management: a review and comparison of 5 current guidelines. Respir Care. 2008; 53:751–767.
5. Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. International Study of Asthma and Allergies in Childhood Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009; 64:476–483.
6. Lai CK, De Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, et al. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003; 111:263–268.
7. Kondo N, Nishimuta T, Nishima S, Morikawa A, Aihara Y, Akasaka T, et al. Japanese pediatric guidelines for the treatment and management of bronchial asthma 2008. Pediatr Int. 2010; 52:319–326.
8. Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention: 2006 revision [Internet]. [place unknown]: Global Initiative for Asthma;2007. cited 2015 Dec 6. Available from: http://www.who.int/respiratory/asthma/GINA_WR_2006_copyright%5B1%5D.pdf.
9. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007; 120:S94–S138.
10. Jusuf L, Hsieh CT, Abad L, Chaiyote W, Chin WS, Choi YJ, et al. Primary care challenges in treating paediatric asthma in the Asia-Pacific region. Prim Care Respir J. 2013; 22:360–362.
11. Chiu KC, Boonsawat W, Cho SH, Cho YJ, Hsu JY, Liam CK, et al. Patients' beliefs and behaviors related to treatment adherence in patients with asthma requiring maintenance treatment in Asia. J Asthma. 2014; 51:652–659.
12. Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004; 114:1282–1287.
13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.
14. Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention: 2014 [Internet]. Global Initiative for Asthma;2007. cited 2016 Jul 8. Available from: http://www.ginasthma.org/2014-GINA-Report%2C-Global-Strategy-for-Asthma-Management-and-Prevention.
15. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.
16. Cho HJ, Jung YH, Yang SI, Lee E, Kim HY, Seo JH, et al. Reference values and determinants of fractional concentration of exhaled nitric oxide in healthy children. Allergy Asthma Immunol Res. 2014; 6:169–174.
17. Song WJ, Kwon JW, Kim EJ, Lee SM, Kim SH, Lee SY, et al. Clinical application of exhaled nitric oxide measurements in a korean population. Allergy Asthma Immunol Res. 2015; 7:3–13.
18. Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008; 32:545–554.
19. Ahn K, Kim J, Kwon HJ, Chae Y, Hahm MI, Lee KJ, et al. The prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in Korean children: nationwide cross-sectional survey using complex sampling design. J Korean Med Assoc. 2011; 54:769–778.
20. Kim HY, Kwon EB, Baek JH, Shin YH, Yum HY, Jee HM, et al. Prevalence and comorbidity of allergic diseases in preschool children. Korean J Pediatr. 2013; 56:338–342.
21. Kim YH, Urm SH, Kim WK. Prevalence of allergic diseases and risk factors in preschool children, 2009. Pediatr Allergy Respir Dis. 2011; 21:165–175.
22. Cho SH, Kim YK, Chang YS, Kim SS, Min KU, Kim YY. Asthma insights and reality in Korea. Korean J Med. 2006; 70:69–77.
23. Wong GW, Kwon N, Hong JG, Hsu JY, Gunasekera KD. Pediatric asthma control in Asia: phase 2 of the Asthma Insights and Reality in Asia-Pacific (AIRIAP 2) survey. Allergy. 2013; 68:524–530.
24. Rabe KF, Adachi M, Lai CK, Soriano JB, Vermeire PA, Weiss KB, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004; 114:40–47.
25. Lang A, Carlsen KH, Haaland G, Devulapalli CS, Munthe-Kaas M, Mowinckel P, et al. Severe asthma in childhood: assessed in 10 year olds in a birth cohort study. Allergy. 2008; 63:1054–1060.
26. Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008; 122:662–668.
27. Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005; 115:132–138.
28. Kim E, Kim MJ, Lee JS, Yoon JS. Association between autumnal exacerbation and dermatophagoides pteronyssinus specific IgE in childhood asthma. Pediatr Allergy Respir Dis. 2007; 17:242–248.
29. Graham LM. Classifying asthma. Chest. 2006; 130:13S–20S.
30. Moon S, Shin J. Performance of universal health insurance: lessons from South Korea. World Health Popul. 2007; 9:95–113.
31. Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. J Asthma. 2003; 40:93–101.
32. Papi A, Corradi M, Pigeon-Francisco C, Baronio R, Siergiejko Z, Petruzzelli S, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013; 1:23–31.
33. Bisgaard H, Le Roux P, Bjåmer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006; 130:1733–1743.
34. Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011; 377:650–657.
35. Yawn BP, Brenneman SK, Allen-Ramey FC, Cabana MD, Markson LE. Assessment of asthma severity and asthma control in children. Pediatrics. 2006; 118:322–329.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download