1. Bousquet J, Reid J, van Weel C, Baena Cagnani C, Canonica GW, Demoly P, et al. Allergic rhinitis management pocket reference 2008. Allergy. 2008; 63:990–996. PMID:
18691301.

2. Sritipsukho P. Aeroallergen sensitivity among Thai children with allergic respiratory diseases: a hospital-based study. Asian Pac J Allergy Immunol. 2004; 22:91–95. PMID:
15565944.
3. Han DH, Ahn JC, Mun SJ, Park SK, Oh SY, Rhee CS. Novel risk factors for allergic rhinitis in Korean elementary school children: ARCO-kids phase II in a community. Allergy Asthma Immunol Res. 2015; 7:234–240. PMID:
25749770.

4. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126:466–476. PMID:
20816182.
5. Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013; 111:465–507. PMID:
24267359.

6. Bouic PJ. A review of the efficacy and safety of Nasaleze™ in the prevention and management of allergic rhinitis. Open Allergy J. 2008; 1:1–4.
7. Diethart B, Emberlin JC, Lewis R. Hydroxypropylmethylcellulose gel application delays Der p 1 diffusion in vitro. Nat Sci (Irvine). 2010; 2:79–84.

8. Åberg N, Dahl Å, Benson M. A nasally applied cellulose powder in seasonal allergic rhinitis (SAR) in children and adolescents; reduction of symptoms and relation to pollen load. Pediatr Allergy Immunol. 2011; 22:594–599. PMID:
21645117.

9. Emberlin JC, Lewis RA. A double blind, placebo controlled trial of inert cellulose powder for the relief of symptoms of hay fever in adults. Curr Med Res Opin. 2006; 22:275–285. PMID:
16466599.

10. Aivazis V, Bourli E, Maratou E, Mavroudi A, Fountzila E, Aivazi D, et al. Study of mucociliary clearance and peak nasal inspiratory flow rate in children with allergic rhinitis before and after therapy with natural cellulose powder. In : Proceedings of the World Allergy Congress 2005; 2005 Jun 26–Jul 1; Munich, Germany. [place unknown]: Nea Pediatrica Chronica;2005.
11. Åberg N, Ospanova ST, Nikitin NP, Emberlin J, Dahl Å. A nasally applied cellulose powder in seasonal allergic rhinitis in adults with grass pollen allergy: a double-blind, randomized, placebo-controlled, parallel-group study. Int Arch Allergy Immunol. 2014; 163:313–318. PMID:
24852424.

12. Emberlin JC, Lewis RA. A double blind, placebo-controlled cross over trial of cellulose powder by nasal provocation with Der p1 and Der f1. Curr Med Res Opin. 2007; 23:2423–2431. PMID:
17767803.

13. Pipkorn U. Budesonide and nasal allergen challenge testing in man. Allergy. 1982; 37:129–134. PMID:
7137521.

14. Dolovich J, Moote DW, Mazza JA, Clermont A, PetitClerc C, Danzig M. Efficacy of loratadine versus placebo in the prophylactic treatment of seasonal allergic rhinitis. Ann Allergy. 1994; 73:235–239. PMID:
8092558.
15. Calderon MA, Bernstein DI, Blaiss M, Andersen JS, Nolte H. A comparative analysis of symptom and medication scoring methods used in clinical trials of sublingual immunotherapy for seasonal allergic rhinitis. Clin Exp Allergy. 2014; 44:1228–1239. PMID:
24773171.

16. Amar SM, Harbeck RJ, Sills M, Silveira LJ, O'Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: monotherapy versus combination in a multiallergen extract. J Allergy Clin Immunol. 2009; 124:150–156. 156.e1–156.e5. PMID:
19523672.

17. Scadding GW, Hansel TT, Durham SR. Nasal provocation testing. In : Adkinson NF, Bochner BS, editors. Middleton's allergy: principle and practice. 8th ed. Philadelphia (PA): Elsevier;2014. p. 652–663.
18. Tantilipikorn P, Vichyanond P, Lacroix JS. Nasal provocation test: how to maximize its clinical use? Asian Pac J Allergy Immunol. 2010; 28:225–231. PMID:
21337904.
19. Linder A. Symptom scores as measures of the severity of rhinitis. Clin Allergy. 1988; 18:29–37. PMID:
3349590.

20. Özgür A, Arslanoğlu S, Etıt D, Demıray U, Önal HK. Comparison of nasal cytology and symptom scores in patients with seasonal allergic rhinitis, before and after treatment. J Laryngol Otol. 2011; 125:1028–1032. PMID:
21791158.

21. Howarth PH, Persson CG, Meltzer EO, Jacobson MR, Durham SR, Silkoff PE. Objective monitoring of nasal airway inflammation in rhinitis. J Allergy Clin Immunol. 2005; 115:S414–S441. PMID:
15746881.

22. Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015; 351:h4672. PMID:
26341898.

23. Sapp M. Research design. In : Sapp M, editor. Basic psychological measurement, research designs, and statistics without math. Springfield (IL): Charles C. Thomas Publisher;2006. p. 74–89.
24. Valerieva A, Popov TA, Staevska M, Kralimarkova T, Petkova E, Valerieva E, et al. Effect of micronized cellulose powder on the efficacy of topical oxymetazoline in allergic rhinitis. Allergy Asthma Proc. 2015; 36:e134–e139. PMID:
26133030.

25. Popov TA, Valerieva A, Church MK, Staevska M, Kralimarkova T, Petkova E, et al. Real-life study on the effect of micronized cellulose powder as add-on to intranasal as-needed treatment of subjects with pollen allergic rhinitis. J Allergy Clin Immunol. 2016; 137(Suppl):AB402.

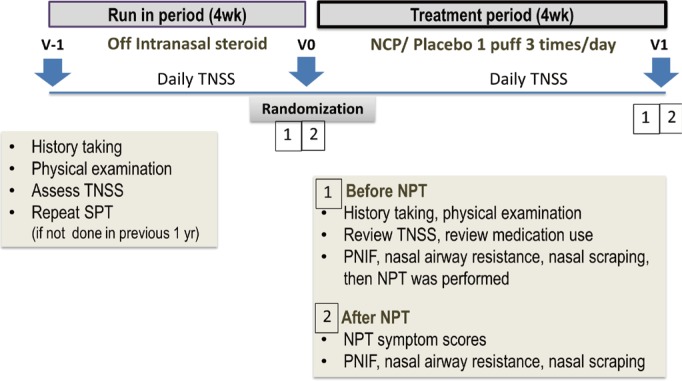
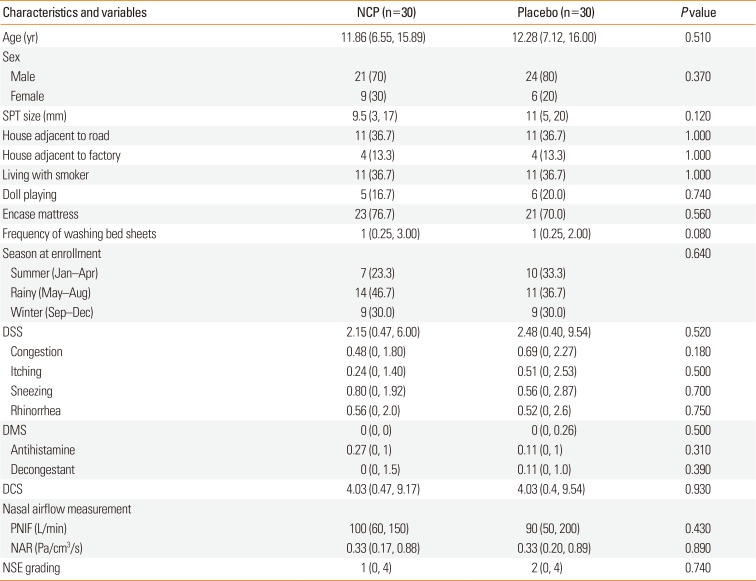
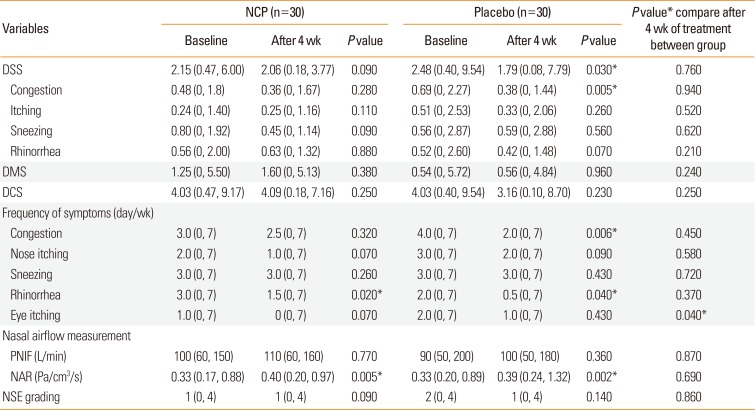
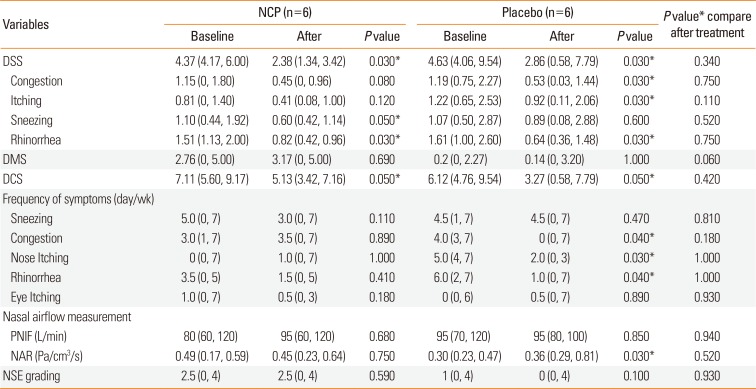




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download