Abstract
Purpose
Comparisons of the characteristics of chronic obstructive pulmonary disease (COPD) and asthma-COPD overlap syndrome (ACOS) have been the focus of several studies since the diseases were defined by the Global Initiative for Asthma and Global Initiative for Chronic Obstructive Lung Disease guidelines. However, no consensus is available yet. In this study, we aimed to compare the characteristics of asthma-COPD overlap (ACO) and COPD.
Methods
We retrospectively reviewed 1,504 patients with COPD in a Korean COPD Subtype Study cohort. The occurrence of ACO was defined as a positive response to a bronchodilator (an increase in forced expiratory volume in 1 second [FEV1] of 12% and 200 mL).
Results
Among 1,504 patients with COPD, 223 (14.8%) were diagnosed with ACO. Men (95.5%) and current smokers (32.9%) were more prevalent in the ACO group compared with the pure COPD group (90.5% and 25.3%, respectively; P=0.015 and P=0.026, respectively). Patients with ACO had a better quality of life (St. George's Respiratory Questionnaire for COPD score=31.0±18.0 [mean±standard deviation]) than those with pure COPD (35.3±19.1) (P=0.002). Although the prevalence of acute exacerbation was not different between the 2 groups, patients with severe exacerbation required hospital admission significantly more frequently in the pure COPD group than in the ACO group. Patients with ACO showed a higher likelihood of FEV1 recovery than those with pure COPD (P<0.001).
Asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome (ACOS) has been defined as a condition characterized by several features of asthma and COPD by the Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) committees in 2014. COPD is characterized by irreversible airflow limitation associated with aging and smoking, whereas asthma is characterized by reversible airflow limitation associated with atopic features.123 Asthma is a completely different disease from COPD in its pathophysiology;4 Therefore, many studies have reported that ACOS is a distinct phenotype of COPD.567 However, there has been no consensus regarding how different ACOS is from COPD in its clinical characteristics and prognosis. Many studies have reported that patients with ACOS have more severe respiratory symptoms, a more greatly impaired quality of life, more frequent exacerbations, and more comorbidities compared with patients with COPD.8910 However, some studies have reported no significant difference between them.5 Moreover, other recent studies reported that patients with ACOS had better prognoses, including mortality and lung function.1112
We analyzed data from a Korean COPD Subtype Study (KOCOSS) cohort collected from 45 study centers throughout Korea.14 Recruitment, enrollment, and measurement occurred between December 2011 and October 2016. Enrollment criteria for the KOCOSS cohort were Korean adults ≥40 years old with a post-bronchodilator (BD) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) value <0.7 at any clinical visit. Exclusion criteria were subjects who (1) could not perform the pulmonary function test, (2) used systemic steroids because of other underlying diseases, and (3) could not communicate with clinicians. Smoking history and status, respiratory symptoms including coughing and sputum, and comorbidities were assessed at the first visit. All data were collected by trained nurses, and patients were followed up at 6-month regular intervals. A pulmonary function test was performed for all subjects and blood sampling was performed at the first visit. The total immunoglobulin E (IgE) level and absolute eosinophil count were analyzed from the obtained blood samples.
Among the 1,675 patients enrolled in the KOCOSS cohort, 1,504 were diagnosed with COPD based on a pulmonary function test with a post-BD FEV1/FVC <0.7 at the first visit. The occurrence of ACO was defined as a positive response to a BD (an increase in FEV1 of 12% and 200 mL). Then, 1,281 patients were diagnosed with “pure” COPD and 223 patients were diagnosed with ACO (Fig. 1).
Among them, 834 were followed up for more than 2 years and analyzed to define exacerbation rate for 2 years. Additionally 189 patients were followed up with pulmonary function tests at 1-year intervals for more than 3 years and analyzed to define change in lung function for 3 years.
The CAT score and St. George Respiratory Questionnaire for COPD patients (SGRQ-C) score were used to assess health status in patients with COPD. The CAT consists of 8 items and includes questions on symptoms, energy, sleep, and activity. A higher score indicates more severe symptoms.15 The SGRQ-C includes 40 items and contains 3 parts scoring symptoms, activities, and impacts on daily life. Total and component scores were calculated according to algorithms provided in the SGRQ-C instruction manual. A higher score means a poorer quality of life.16
Exacerbation was defined as the worsening of any respiratory symptom, such as increased sputum volume, purulence, or increased dyspnea, which required treatment with systemic corticosteroids, antibiotics, or both. Exacerbation history in the previous 12 months was assessed at the first visit, the 1-year follow-up visit, and the 2-year follow-up visit.
For continuous variables, descriptive statistics are reported as means with standard deviations (SDs), and for categorical variables as the number of patients per category and the frequency of responses. Comparisons of continuous variables were made using the 2-sample t test; the χ2 test or Fisher's exact test were used for comparisons of categorical variables. Comparisons of FEV1 changes over time were assessed with a repeated analysis of variance (ANOVA). Differences were considered statistically significant at P<0.05.
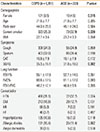
Among 1,504 patients with COPD, 223 (14.8%) were diagnosed with ACO after presenting a bronchodilator response (BDR). The pure COPD group had more women (9.5%) than the ACO group (4.5%; P=0.015). The ACO group had more current smokers (32.9%) than the pure COPD group (25.3%; P=0.026). Although the prevalences of coughing and sputum were not different between the 2 groups, patients with ACO reported a better quality of life (SGRQ-C score=31.0±18.0 [mean±standard deviation]) than those with pure COPD (35.3±19.1) (P=0.002). There was no significant difference in lung function or comorbidities between the 2 groups (Table 1). Although the data are not shown, the prevalence of a treatment-naïve status (7.7% and 11.6%), mean duration of treatment for COPD (5.3±5.3 and 4.9±4.6 years), or prevalence of a history of inhaled corticosteroids (ICS) use (83.1% and 82.5%) was not significantly different between the COPD and ACO groups.
Among all the patients, 834 (55.4%) were followed up for more than 2 years. At baseline, 27.5% of pure COPD subjects and 20.3% of ACO subjects experienced an acute exacerbation in the previous year (P=0.086). After 1 year, 20.9% of pure COPD subjects and 18.0% of ACO subjects responded that they had an acute exacerbation in the previous year (P=0.461). After 2 years, 19.7% of the pure COPD subjects and 14.3% of the ACO subjects experienced an acute exacerbation in the previous year (P=0.144). Pure COPD subjects showed more frequent acute exacerbations than did ACO subjects; however, there was no statistically significant difference (Fig. 2A).
Among the 834 patients with more than 2 years of follow-up data, 12.7% of pure COPD subjects and 8.3% of ACO subjects experienced severe acute exacerbations requiring hospital admission in the previous year at baseline (P=0.150). In the results obtained after the first year, pure COPD subjects more frequently had severe acute exacerbations requiring hospital admission (5.5%) in the previous year than ACO subjects (1.5%; P=0.030). In the results from after the second year, the same findings were observed (5.6% vs 0.8%; P=0.015) (Fig. 2B). Among pure COPD subjects with acute exacerbations, 26.5% were admitted to the hospital. ACO subjects were admitted less frequently (16.7%); however, there was no significant difference (P=0.051) (Fig. 2C).
Among all patients, 189 were followed up for more than 3 years and underwent pulmonary function tests at 1-year intervals. ACO subjects tended to recover pulmonary function over time (predicted FEV1: 50.2% at baseline; 58.3% after 1 year; 57.6% after 2 years; and 59.1% after 3 years). However, pulmonary function was not recovered in pure COPD subjects (predicted FEV1: 54.1% at baseline; 55.9% after 1 year; 56.0% after 2 years; and 53.8% after 3 years). The difference in this trend was statistically significant between the 2 groups (P<0.001) (Fig. 3A). Moreover, the changes in absolute FEV1 showed the same results as those for predicted FEV1 (P<0.001) (Fig. 3B).
We attempted to find predictive variables for ACO. Levels of IgE (number of included patients, 265; odds ratio [OR], 0.999; P=0.177), history of asthma (number of included patients, 1,477; OR, 1.152; P=0.334), and history of allergic rhinitis (number of patients included, 1,475; OR, 1.107; P=0.662) were not significant predictive factors. However, the AEC was a significant predictive factor for ACO (number of patients included, 1,199). We analyzed the predictive power of the AEC for ACO according to various AEC cutoff values. When we used ≥200/µL as a cutoff value, the OR was 1.580 (95% confidence interval, 1.150-2.17; P=0.005). The sensitivity and specificity were 51.4% and 59.9%, respectively (P=0.015). When we used a cutoff value of ≥250/µL, the OR was significant, whereas the sensitivity and specificity were not significantly predictive. The results from a cutoff value of ≥300/µL were not significantly predictive. Although the results from a cutoff value of ≥500/µL were not significantly predictive, the specificity was the highest (90.3%) (Table 2).
There are many studies showing that ACO had a poor prognosis compared with pure COPD. However, this cohort study determined that ACO is a distinct phenotype of COPD representing better clinical outcomes. First, we observed that patients with ACO had the possibility of recovering their lung function. In fact, the baseline lung function at the first visit was poorer in patients with ACO than that in those with COPD in this cohort study. However, the decline in lung function was more remarkable in patients with pure COPD than those with ACO in this cohort study. Some cross-sectional studies reported that patients with ACO demonstrated poorer lung function than those with pure COPD.17 However, Kauppi et al.18 reported that the post-BD FEV1 was significantly better in ACO (67.4%) than in pure COPD (61.4%). In addition, De Marco et al.11 showed a rapid decline in FEV1 in pure COPD (-7.64 mL/year) in contrast to an improvement in FEV1 in ACO (1.62 mL/year), which is consistent with our result. The recovery of lung function in ACO might be due to an asthma component with a good response to ICS treatment. Thus, we suggest that the possibility of recovering lung function may be the same characteristics of ACO compared as pure COPD.
Second, we revealed that the severe exacerbation rate was lower in ACO compared with pure COPD. Some studies reported that the exacerbation rate was higher in ACO than in COPD.510 This cohort study showed similar total exacerbation rates between ACO and pure COPD. However, we showed that severe exacerbation requiring hospital admission was more frequent among patients with pure COPD. This result may be associated with the results of the previous studies which described that patients with COPD had a lower chance of recovering lung function. Izquierdo-Alonso et al.19 previously showed that the exacerbation rate was lower in ACO (64.9%) compared with COPD of the emphysema type (68.8%). In addition, they showed that the number of visits to the emergency department was slightly lower in ACO (0.79/year) compared with COPD of the emphysema type (1.12/year) and COPD of the bronchitis type (1.25/year); however, the difference was not statistically significant. The higher survival rate among ACO patients was confirmed by Cosio et al.12 and this may be understood in the same context as in this cohort study. Further studies are needed to differentiate severe exacerbation rates between ACO and COPD.
Finally we showed that the symptoms were better in ACO compared pure COPD. Many studies reported that symptoms and quality of life were worse in ACO compared with pure COPD.8182021 Some studies showed that symptoms and quality of life in ACO were not different from those in COPD.5 It was difficult to find studies showing better symptoms in ACO, in concordance with this cohort study. Although data are not shown, SGRQ-C was significantly correlated with exacerbation rate and lung function recovery. Multivariate analysis showed that ACO was a dependent prognostic factor on SGRQ-C for exacerbation and lung function recovery. Then, we can suggest that subjects with ACO have less severe symptoms, and therefore it might lead to rare severe exacerbation, and the possibility of lung function recovery.
The result that ACO showed better clinical outcomes than pure COPD in this cohort study is in contrast to major opinions supported by extensive studies. The main reason is due to the differences in study design. This cohort study included treatment-naïve and ICS-naïve subjects. Moreover, the mean treatment duration was relatively short (4-5 years) compared with those in other studies (usually more than 10 years). These might have caused ACO to show better clinical outcomes after enrollment in the cohort study followed by proper ACO management. Another reason is due to the unique asthma characteristics in Korea. Asthma is diagnosed and managed mainly in primary care. In Korea, the prevalence of asthma is estimated to be about 3%-5%, whereas it is thought to be 8%-20% in other countries. Many Korean asthmatic patients do not use ICS treatment properly (the treatment rate is lower than 50%), especially in primary care.22 Moreover, the asthma fatality rate per 100,000 asthmatics is 7%-9% in Korea, whereas it is less than 5% in most countries. This means that many primary clinics missed the diagnosis of asthma, and even physician-diagnosed asthma patients do not use their treatment drug properly. These unique asthma characteristics in Korea might have induced the ACO subjects to have a lesser opportunity to treat their asthma properly prior to enrollment, and finally, their prognoses improved after enrollment in the cohort study which consisted of pateints from second or tertiary hospitals following proper ACO management, including ICS treatment.
ACOS has recently received attention, and the definition of ACOS has not been established. The Spanish criteria,23 modified Spanish criteria,12 and European Respiratory Journal (ERJ) criteria24 are the most prominent criteria, and require that various items be fulfilled to diagnose ACOS. The Spanish criteria were created in 2012 and include eosinophilia in sputum, a history of atopy, and the level of total IgE. However, in practice, many institutes cannot assess eosinophilia in sputum. The modified Spanish criteria and ERJ criteria were created in 2016 and exclude sputum eosinophilia, and instead include blood eosinophilia. However, these criteria still contain 6 items. Many studies have attempted to diagnose ACOS simply and properly;25 however, there is still no consensus. Caillaud et al.5 defined ACOS very simply as follows: a patient enrolled in a COPD cohort study who was diagnosed with asthma by a physician before the age of 40 years. We used very simplified criteria for ACO: the intersection of asthma and COPD, as defined by the 2014 GINA and GOLD guidelines, with a BDR (post-BD FEV1 improvements of 12% and 200 mL).
Some researchers believe that a BDR is not credible to identify the phenotype of COPD, ACO.26 However, there are many studies that used simplified criteria for ACOS as this study did (in the assumptions on COPD). Fu et al.6 (BDR or airway hyper-responsiveness), Menezes et al.17 (BDR and wheezing), Kauppi et al.18 (BDR including PEF and an exercise test), and Contoli et al.27 (BDR and a history of asthma) used relatively simple criteria, including BDR and/or other simple conditions. Recently, Baarnes et al.28 showed similar characteristics in ACOS defined by wheezing, BDR, or both, and they concluded that ACOS could be identified only by BDR as in this cohort study. In addition, considering the prevalence of ACOS among COPD, which is 15%-25% as defined by various criteria,5293031 the prevalence of ACOS in this study (14.8%) indicates that the definition used in this study does not differ substantially from the more complicated classic criteria previously described. Thus, we thought that the simplified criteria for ACOS used in this study could be applied. However, some researchers still have questions whether COPD with mild BDR is sufficient to define ACOS. Barnes9 described that ACO is better to include the various phenotypes of status with features of COPD and asthma: COPD with mild BDR, COPD with eosinophilic inflammation, asthma with smoking history, and asthma with neutrophilic inflammation. Furthermore,the 2017 GINA guideline also used the term of ACO.13 Then, we used the term of ACO described by Barnes and the 2017 GINA guideline rather than ACOS to avoid controversy.
When we attempted to find predictive variables for ACO, blood eosinophil count was significantly superior to the level of total IgE, history of asthma, and history of allergic rhinitis. Moreover, the most powerful cutoff value for eosinophil count was 200/µL. The modified Spanish criteria and ERJ criteria contain a history of asthma as a major criterion, whereas they include blood eosinophil count as a minor criterion; the cutoff values for blood eosinophilia were 5% and 300/µL in the modified Spanish criteria and ERJ criteria, respectively. We suggest that the blood eosinophil count will be a better predictive marker for ACO than expected. Additionally, the adjusted cutoff value for blood eosinophilia can be applied to stronger predictive power in ACO.
This study has several limitations. First, ACO as defined in this study might not be the correct definition of ACO. Additionally, we could not apply the definition previously described (GINA and GOLD guidelines, Spanish criteria, modified Spanish criteria, and ERJ criteria), as this cohort study did not contain all the parameters described in those criteria. Second, only 50% and 13% of subjects were followed up for 2 years and 3 years, respectively, and they were analyzed for exacerbation and lung function. Almost all excluded patients in the follow-up study were recently enrolled patients in the cohort study. However, we cannot exclude a selection bias in which subjects who were more compliant might have been enrolled in larger numbers in the follow-up study. Third, there were some missing values for the level of total IgE, blood eosinophil count, history of asthma, and history of allergic rhinitis. Moreover, a history of asthma should be changed to a history of asthma before the age of 40 years for better evaluation. Finally we cannot fully exclude the possibility of selection bias. The results that ACO patients showed less symptoms might be brought about by selection bias. KOCOSS cohort data were collected from 45 institutions across the country for 5 years. The recruitment and enrollment of subjects are still ongoing, and it is necessary to review the results of the re-analysis of the long-term follow-up data.
This COPD cohort study defined ACO (14.8%), which is a distinct type of COPD. We suggest that subjects with ACO have less severe symptoms, and therefore it might lead to rare severe exacerbation and the possibility of lung function recovery. Moreover, blood eosinophil count (≥200/µL) could be a suitable predictive factor for ACO.
Figures and Tables
Fig. 1
Study flow. KOCOSS, Korean Chronic Obstructive Pulmonary Disease Subtype Study; COPD, chronic obstructive pulmonary disease; ACO, asthma-chronic obstructive pulmonary disease overlap.
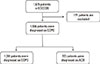
Fig. 2
Comparisons of (A) exacerbation rates, (B) severe exacerbation rates required hospitalization, and (C) prevalence of severe exacerbation, between COPD and ACO. COPD, chronic obstructive pulmonary disease; ACO, asthma-chronic obstructive pulmonary disease overlap.
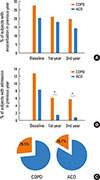
Fig. 3
Comparison of pulmonary function trends between COPD and ACO. Predicted FEV1 (A) and absolute FEV1 (B) are shown. COPD, chronic obstructive pulmonary disease; ACO, asthma-chronic obstructive pulmonary disease overlap; FEV1, forced expiratory volume in 1

Table 1
Comparison of demographics and clinical characteristics between COPD and ACO
Data are shown as mean ± standard deviation or number (%).
COPD, chronic obstructive pulmonary disease; ACO, asthma-chronic obstructive pulmonary disease overlap; BMI, body mass index; CAT, chronic obstructive pulmonary disease assessment test; SGRQ, St. George Respiratory Questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HTN, hypertension; DM, diabetes mellitus; MI, myocardial infarction; HF, heart failure.
Table 2
Predictive power of AEC for ACO

References
1. Yoon HI. Respiratory review of 2014: asthma. Tuberc Respir Dis. 2014; 77:237–242.
2. Kim DK, Park YB, Oh YM, Jung KS, Yoo JH, Yoo KH, et al. Korean Asthma Guideline 2014: summary of major updates to the Korean Asthma Guideline 2014. Tuberc Respir Dis. 2016; 79:111–120.
3. Seo JY, Hwang YI, Mun SY, Kim JH, Kim JH, Park SH, et al. Awareness of COPD in a high risk Korean population. Yonsei Med J. 2015; 56:362–367.
4. Kim HY. Resveratrol in asthma: a French paradox? Allergy Asthma Immunol Res. 2017; 9:1–2.
5. Caillaud D, Chanez P, Escamilla R, Burgel PR, Court-Fortune I, Nesme-Meyer P, et al. Asthma-COPD overlap syndrome (ACOS) vs ‘pure’ COPD: a distinct phenotype? Allergy. 2017; 72:137–145.
6. Fu JJ, McDonald VM, Gibson PG, Simpson JL. Systemic inflammation in older adults with asthma-COPD overlap syndrome. Allergy Asthma Immunol Res. 2014; 6:316–324.
7. Lee HY, Kang JY, Yoon HK, Lee SY, Kwon SS, Kim YK, et al. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014; 55:980–986.
8. Miravitlles M, Soriano JB, Ancochea J, Muñoz L, Duran-Tauleria E, Sánchez G, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013; 107:1053–1060.
9. Barnes PJ. Asthma-COPD overlap. Chest. 2016; 149:7–8.
10. Chung WS, Lin CL, Kao CH. Comparison of acute respiratory events between asthma-COPD overlap syndrome and COPD patients: a population-based cohort study. Medicine (Baltimore). 2015; 94:e755.
11. de Marco R, Marcon A, Rossi A, Antó JM, Cerveri I, Gislason T, et al. Asthma, COPD and overlap syndrome: a longitudinal study in young European adults. Eur Respir J. 2015; 46:671–679.
12. Cosio BG, Soriano JB, López-Campos JL, Calle-Rubio M, Soler-Cataluna JJ, de-Torres JP, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016; 149:45–52.
13. Global Initiative for Asthma. 2017 GINA report, global strategy for asthma management and prevention [Internet]. place unknown: Global Initiative for Asthma;2017. cited 2017 Mar 21. Available from: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/.
14. Lee JY, Chon GR, Rhee CK, Kim DK, Yoon HK, Lee JH, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: the Korea COPD subgroup study team cohort. J Korean Med Sci. 2016; 31:553–560.
15. Tsiligianni IG, van der Molen T, Moraitaki D, Lopez I, Kocks JW, Karagiannis K, et al. Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). BMC Pulm Med. 2012; 12:20.
16. Jones PW. St George's respiratory questionnaire for COPD patients (SGRQ-C): manual. London: St Geroge's University of London;2005.
17. Menezes AM, Montes de Oca M, Pérez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014; 145:297–304.
18. Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpeläinen M, Kinnula VL, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011; 48:279–285.
19. Izquierdo-Alonso JL, Rodriguez-Gonzálezmoro JM, de Lucas-Ramos P, Unzueta I, Ribera X, Antón E, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Respir Med. 2013; 107:724–731.
20. Chung JW, Kong KA, Lee JH, Lee SJ, Ryu YJ, Chang JH. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2014; 9:795–804.
21. Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011; 12:127.
22. Kim SY, Jung JY, Park MS, Kang YA, Kim EY, Kim SK, et al. Increased prevalence of self-reported asthma among Korean adults: an analysis of KNHANES I and IV data. Lung. 2013; 191:281–288.
23. Soler-Cataluña JJ, Cosío B, Izquierdo JL, López-Campos JL, Marín JM, Agüero R, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012; 48:331–337.
24. Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016; 48:664–673.
25. Barrecheguren M, Román-Rodríguez M, Miravitlles M. Is a previous diagnosis of asthma a reliable criterion for asthma-COPD overlap syndrome in a patient with COPD? Int J Chron Obstruct Pulmon Dis. 2015; 10:1745–1752.
26. Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respir Med. 2013; 1:564–573.
27. Contoli M, Baraldo S, Marku B, Casolari P, Marwick JA, Turato G, et al. Fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease: 5-year follow-up. J Allergy Clin Immunol. 2010; 125:830–837.
28. Baarnes CB, Kjeldgaard P, Nielsen M, Miravitlles M, Ulrik CS. Identifying possible asthma-COPD overlap syndrome in patients with a new diagnosis of COPD in primary care. NPJ Prim Care Respir Med. 2017; 27:16084.
29. de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013; 8:e62985.
30. Tamada T, Sugiura H, Takahashi T, Matsunaga K, Kimura K, Katsumata U, et al. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015; 10:2169–2176.
31. Cosio BG, Soriano JB, López-Campos JL, Calle M, Soler JJ, De-Torres JP, et al. Distribution and outcomes of a phenotype-based approach to guide COPD management: results from the CHAIN cohort. PLoS One. 2016; 11:e0160770.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download