INTRODUCTION
The geriatric population is increasing worldwide and the burden of asthma is more significant in elderly asthmatics compared to younger ones. The number of elderly people is projected to reach 20% and 36% of total populations of the United States and China, respectively.
12 The prevalence of asthma is increasing at all ages, and it is reported to be 5% to 10% in elderly people, similar to younger ones.
13 A main burden of elderly asthma is attributed to asthma severity, hospitalization, near-fatal asthma related-events, and medical costs.
245 To reduce the severity, mortality, and burden of asthma in elderly asthmatics, assessment of asthma control is mandatory.
Asthma control is assessed in terms of symptom control and future risk for asthma exacerbation (AE).
6 Global Initiative for Asthma (GINA) symptom control tool, Asthma Control Questionnaire, and Asthma Control Test (ACT) are validated methods for assessing symptom control. To perform these tools for symptom control, patients have to recall their symptoms, including day/night time symptoms, reliever use, and activity limitation during the previous 1 to 4 weeks. One of the most important risk factors for AE is the history of ≥1 exacerbation in the previous year; therefore, patients should recall their AE history to evaluate the future risk for AE.
6 Spirometry which is recommended in assessing the future risk for AE requires proper coordination, and cognitive function and effort.
1 The tools for asthma control recommended by the current guideline
6 are dependent on cognitive function and effort which is substantially impaired in elderly. In addition, assessment of asthma symptoms, especially dyspnea, may be inappropriate for elderly because of multiple comorbidities that can cause dyspnea in elderly. Therefore, objective assessment tool which is significantly associated with asthma control status and AE in elderly subjects are needed.
MATERIALS AND METHODS
Asthmatics aged >60 years who were diagnosed with asthma for more than 6 months and treated with step 2 or 3 by the GINA guidelines were enrolled. Subjects were recruited between August 2011 and February 2012 from 4 university hospitals in Korea.
7 This study was approved by Ajou University Institutional Review Board (IRB No. GEN-CT4-10-095), and the other 4 hospitals approved the study. Informed consent was obtained from each patient. During the 12-week study period, the subjects used either 400 µg of inhaled budesonide plus 10 mg of montelukast or 800 µg of inhaled budesonide. The occurrence of AE during the 4-week run-in (maintaining with inhaled budesonide at 400 µg per a day) and 12-week treatment period was monthly monitored, which was defined when one of the following criteria was satisfied: use of systemic oral corticosteroids, asthma-related unscheduled visit/emergency department visit/hospitalization, and use of reliever medication. For the overall study period, all study subjects wrote a diary and the completeness was calculated as a compliance. After 12 weeks of antiasthmatic medication (maintaining same dose), the plasma levels of the metabolites leukotriene E
4 (LTE
4) and prostaglandin F
2α (PGF
2α) were measured. ACT score calculation, asthma quality of life (AQoL) assessment, sputum eosinophil counts, peripheral eosinophil counts, and pulmonary function tests were performed at week 12. The study subjects were divided into group 1 (asthmatics who experienced AE during the study period) and group 2 (those who did not). The LTE
4 and PGF
2α levels were quantified as previously reported.
8 In brief, Mass Hunter Quantitative Analysis B.07.00 (Agilent Technologies, Santa Clara, CA, USA) was used to quantify the LTE
4 and PGF
2 levels in plasma samples. The following deuterated internal standards were used: LTE
4-d5 for LTE
4 and 8-iso PGF
2α-d4 for PGF
2α (Cayman Chemical Company, Ann Arbor, MI, USA). The concentration of each metabolite was determined from calibration curves using linear regression analysis. Correlation coefficients were >0.99 for all metabolites. All the chemicals were of high-performance liquid chromatograph (HPLC) grade. General linear regression analysis was performed to adjust for confounding factors in order to compare the metabolite levels between group 1 and 2 subjects. Receiver operating characteristic (ROC) curve analysis was used to discriminate the groups of patients who experienced AE.
RESULTS
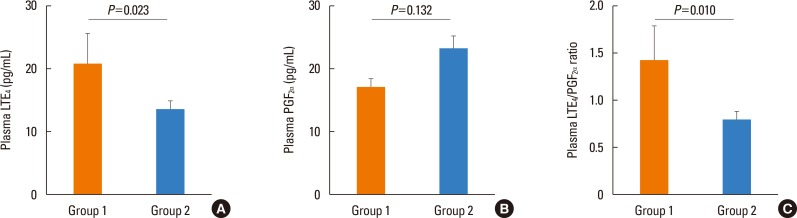
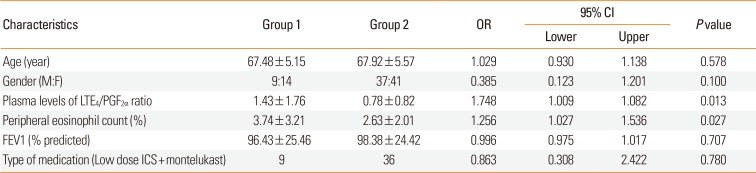
A total of 101 patients aged 60-85 years were enrolled. Twenty-three patients (22.8%) had experienced AE over the study period. Among the group 1 patients, 5 (21.74%), 1 (4.35%), 14 (60.87%), and 3 (13.04%) had experienced the last AE in the first, second, third, and fourth months, respectively. Baseline clinical characteristics are summarized in
Table 1. Group 1 subjects had a significantly higher plasma LTE
4 level and a LTE
4/PGF
2α ratio at week 12 than group 2 subjects (LTE
4 level: 20.83±23.37 vs 13.49±11.04 pg/mL,
P=0.023; LTE
4/PGF
2α ratio: 1.43±1.76 vs 0.79±0.82,
P=0.010) (
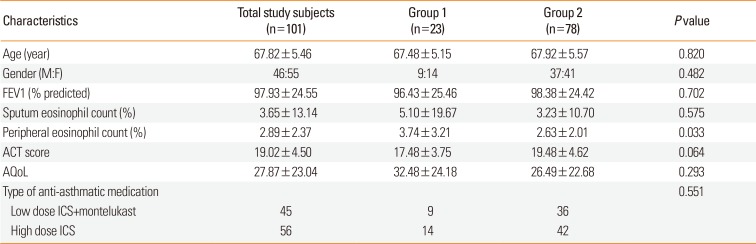
Figure). The plasma PGF
2α level showed no significant difference between group 1 and group 2 subjects (17.17±6.40 vs 23.24±19.13,
P=0.132). The compliance was not significantly different between group 1 and 2 subjects. The plasma LTE
4 level and LTE
4/PGF
2α ratio were not significantly different according to the time that subjects experienced the last AE. The plasma LTE
4 level and LTE
4/PGF
2α ratio showed no significant difference between the patients who had used 400 µg of budesonide plus 10 mg of montelukast and those who had used 800 µg of inhaled budesonide after adjusting for age and gender (LTE
4 level: 15.92±12.04 vs 14.55±17.02 pg/mL,
P=0.436; LTE
4/PGF
2α ratio: 1.00±0.96 vs 0.89±1.26,
P=0.476).
By a binary logistic regression model, the plasma LTE
4/PGF
2α ratio and peripheral eosinophil count (%) at week 12 were significantly associated with AE during the study period (odds ratio [OR]=1.748,
P=0.013; OR=1.256,
P=0.027, respectively) (
Table 2). ROC curves were generated to discriminate the asthmatics with previous AE from those without previous AE using a logistic regression model for age, gender, plasma LTE
4/PGF
2α ratio, and peripheral eosinophil count (%) at week 12. The area under the curve was 0.700 (
P=0.004), with 73.9% sensitivity and 47.9% specificity. When ROC curves are generated from the LTE
4 level, LTE
4/PGF
2α ratio or peripheral eosinophil count (%) at week 12, the area under the curve was 0.608, 0.622, and 0.619, respectively.
DISCUSSION
This is the first study to suggest objective assessment tools which is significantly associated with previous AE in elderly asthmatics. A combination of plasma LTE4/PGF2α ratio and peripheral eosinophil count is reliable for assessing previous AE.
Biomarkers including fractional exhaled nitric oxide (FeNO), sputum eosinophilia, and urinary LTE
4, related with future AE have been widely studied.
9 However, objective assessment tools related with previous AE have been rarely studied. The current guideline recommends to treat asthmatics according to asthma control status.
6 The validated tools for assessing asthma control status are symptom control status by GINA, ACT score, asthma control questionnaire, spirometry, and presence of previous AE. Questionnaire items of GINA symptom control, ACT, and asthma control questionnaire all includes reliever usage for previous 1 to 4 weeks. Therefore, we included reliever use in the definition of AE in the present study which is more relevant to assess asthma control status on the basis of real life in clinical practice. These tools are recommended to be applied to elderly asthmatics as in younger asthmatics, though they are substantially cognitive function- and effort-dependent. Taking into account the characteristics of elderly,
1 objective tools for assessing asthma control status, such as, previous AE are needed. In the present study, although the ACT score and AQoL were not significantly different whether the patients experienced AE or not, the plasma LTE
4/PGF
2α ratio and peripheral eosinophil count at week 12 were significantly associated with AE using a binary logistic regression model. With the ROC curve to discriminate asthmatics with previous AE, a combination of 2 parameters had 73.9% sensitivity and 47.9% specificity. Taken together, the plasma LTE
4/PGF
2α ratio and peripheral eosinophil count may be potential tools for assessing asthma control status along with GINA symptom control, ACT, and asthma control questionnaire, especially for elderly asthmatics.
LTE
4 mediates inflammatory cell infiltration, mucus secretion, vascular permeability, and smooth muscle contraction, and plays an important role in asthma pathogenesis.
10 Previous studies demonstrated that the increase in the LTE
4 level during acute AE were reversed after the resolution of AE.
1112 In a previous study,
11 urinary LTE
4 was measured in asthmatic children during an acute exacerbation and 1 month later and the levels were compared. The authors demonstrated that the production of urinary LTE
4 was significantly higher than normal controls not only during AE, but also 1 month later after AE. In the present study, increased plasma LTE
4 level and LTE
4/PGF
2α ratio were noted in patients with previous AE during the previous 16-week period. Similar to the previous study,
11 our study showed no significant difference in the plasma LTE
4 level according to the time point of the last AE, suggesting that plasma LTE
4 level may represent an ongoing inflammatory process rather than acute inflammatory response and can be applied to assess asthma control status. Considering that plasma collection is convenient and that metabolite measurement is an objective method, plasma LTE
4/PGF
2α ratio representing dysregulation of arachidonic acid metabolism can be useful for assessing an episode of previous AE.
The plasma LTE
4 level and LTE
4/PGF
2α ratio showed no significant difference between the patients who used 400 µg of budesonide plus 10 mg of montelukast and those who used 800 µg of inhaled budesonide. Cysteinyl LTs are known to be not completely suppressed by treatment with corticosteroids alone. However, previous studies
1314 demonstrated that not only systemic steroids, but also inhaled corticosteroids (ICSs) decrease cysteinyl LTs concentration. Therefore, it is difficult to directly compare the plasma LTE
4 level and LTE
4/PGF
2α ratio between patients treated with LT receptor antagonists and double dose of ICSs and to evaluate the effect of LT receptor antagonists on the LTE
4 level.
In conclusion, a combination of plasma LTE4/PGF2α ratio and peripheral eosinophil count can be an objective assessment tool which is significantly associated with asthma control status in elderly asthmatics. Further studies in a larger number of patients are needed to validate our results.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download