Abstract
There was no previous population-based study on the comparison of the risk of chronic spontaneous urticaria (CSU) between autoimmune thyroid diseases (AITD) and age- and gender-matched controls. The primary objective of this study was to evaluate the risk of CSU after diagnosis of AITD using national registry data from Korea. The secondary objective was to evaluate other risk factors of CSU. Based on the disease code diagnoses in 2003-2005, we composed an AITD group (n=3,659) and an age- and gender-matched control group (n=18,295). Each patient was tracked for whether CSU occurs or not until 2013. After adjusting for demographic differences and comorbidities, patients with AITD had a significantly higher rate of CSU compared to the control group (hazard ratio [HR], 1.46; 95% confidence interval [CI], 1.25-1.70; P<0.001). Among the AITD patients, the adjusted HR for CSU was higher in patients with Hashimoto's thyroiditis (HR, 1.50) than in those with Grave's disease (HR, 1.33), although the difference was not statistically significant (P=0.368). Analysis of CSU patients associated with AITD showed that female patients had a significantly higher risk of CSU compared to male ones (HR, 1.34; P=0.001) and that those with allergic rhinitis (HR, 1.51; P<0.001), atopic dermatitis (HR, 2.44; P<0.001), and asthma (HR, 1.50; P<0.001) had a significantly higher risk of CSU compared to patients without respective diseases. Our results demonstrated that AITD could be significantly associated with an increased risk of CSU.
Chronic urticaria (CU) is a disorder defined as transient and itchy wheals for more than 6 weeks and is experienced by 0.1% of the general population.1 CU is classified in to chronic spontaneous urticaria (CSU) and inducible urticaria by physical stimuli.2 Up to 45% of patients with CU have immunoglobulin G (IgG) autoantibodies directed against either IgE (5%-10%) or FcεRI (35%-40%).3 Patients with CU are at increased risk of having autoimmune conditions, and it has been hypothesized that the inflammatory processes associated with these autoimmune conditions may lead directly to urticaria or increase the individual's susceptibility to CU.4
Autoimmune thyroid diseases (AITD), of which Grave's disease and Hashimoto's thyroiditis account for the majority of cases, are common autoimmune ones characterized by various degrees of lymphocytic infiltration of the thyroid gland and thyroid autoantibodies.5 Many previous studies have investigated the association between AITD and CSU in a hospital-based design. However, to our knowledge, only 2 population-based studies investigated the association between AITD and CSU, in which they did not use an age- and gender-matched controls.67
The aim of this study was to investigate the relative risk of CSU in the AITD group compared to the control group using national registry data of Korea. The primary objective of this study was to evaluate the adjusted risk of CSU in patients with AITD. The secondary objective was to evaluate other risk factors of CSU, including demographic data, and comorbid metabolic and allergic diseases.
This was a population-based study using the Korean National Health Insurance Service National Sample Cohort 2002-2013 made by the Korean National Health Insurance Service (KNHIS). This database was composed of 1,025,340 nationally representative random patients and included all medical claims from 2002 to 2013.8 KNHIS used the Korean Classification of Diseases (KCD), 6th revision, which was similar to the International Classification of Diseases (ICD), 10th revision.
The AITD group and the age- and gender-matched control group were generated as follows: we excluded patients who were treated for AITD or CSU in 2002, and patients under the age of 20 years. The AITD group included all patients diagnosed as AITD, which included Grave's disease (KCD/ICD code E05.0) and Hashimoto's thyroiditis (E06.3) between 2003 and 2005. The control group was composed of randomly selected patients who were matched to the AITD group according to age and gender at a 1:5 ratio among patients who were not diagnosed with AITD in 2002-2013. A specific ICD-10 code for CSU is lacking. To overcome this limitation, we used previously validated algorithm for defining CSU. We defined a diagnosis of CSU when one of the following 2 criteria is met for each year between 2006 and 2013: (1) either 2 outpatient diagnosis of ICD-10 code L50.1 (idiopathic urticaria), L50.8 (other specified urticaria), or L50.9 (urticaria, unspecified) at least 6 weeks apart; or (2) one outpatient diagnosis of L50.1, L50.8, or L.50.9 plus one diagnosis of T78.3 (angioneurotic edema) at least 6 weeks apart. Cherepanov et al.9 investigated validation in this algorithm identifying patients with CSU and found that it had a positive predictive value of 90.4% and a sensitivity of 71.1%. Since original algorithm was made by ICD-9 code, each disease code was modified to corresponding ICD-10 code. Demographic data and comorbidities were collected for each patient. The patients were grouped according to household income: high-income (20%-100%) and low-income groups (0%-20%). They were also grouped according to the region of residence: the urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, Ulsan, and Gyeonggi-do) and rural groups (Gangwon-do, Chungcheongbuk-do, Chungcheongnam-do, Gyeongsangbuk-do, Gyeongsangnam-do, Jeollabuk-do, Jeollanam-do, and Jeju-do). We defined comorbid metabolic diseases, including type 2 diabetes mellitus (DM), hypertension, and dyslipidemia, in patients who had both 1or more diagnoses and associated prescribed medication between 2003 and 2005. Comorbid allergic diseases, including allergic rhinitis, asthma, and atopic dermatitis, were defined by 1 or more diagnosis codes between 2003 and 2005. Chi-square tests were performed to examine the differences between the AITD and control groups. To identify the hazards associated with CSU, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using univariate and multivariate Cox proportional hazard regression.
Table 1 shows the demographic data and comorbidities of the AITD group (n=3,659) and the control group (n=18,295). In the AITD group, the number of patients with Grave's disease or Hashimoto's thyroiditis was 2,291 (62.6%) or 1,368 (37.4%), respectively. The female gender (80.8%) and age of 40-64 years (52.3%) were predominant in the AITD group. The proportion of patients with the lower 20% income and rural residence group was significantly lower in the AITD group compared with the control group (P<0.001). Regarding comorbidities, the proportions of patients with type 2 DM, hypertension, dyslipidemia, allergic rhinitis, atopic dermatitis, or asthma was significantly higher in the AITD group compared with the control group (P<0.001).
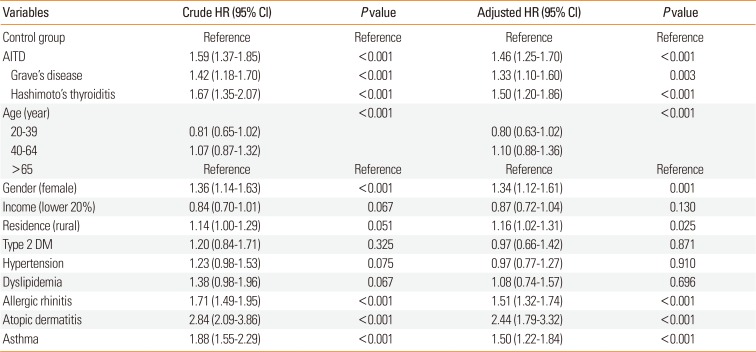
Table 2 shows the HRs for CSU during the 8-year follow-up period using univariate and multivariate Cox regression models. After adjusting for demographic differences and comorbidities, patients with AITD had a significantly higher risk of developing CSU compared with the control group (adjusted HR, 1.46; 95% CI, 1.25-1.70; P<0.001). Among the AITD patients, the adjusted HR for CSU was higher in patients with Hashimoto's thyroiditis (HR, 1.50; 95% CI, 1.20-1.86; P<0.001) than those the patients with Grave's disease (HR, 1.33; 95% CI, 1.10-1.60; P=0.003), although the difference was not statistically significant (P=0.368). There was no significantly higher risk of developing CSU in the age groups of 20-39 (HR, 0.80; 95% CI, 0.63-1.02) and 40-64 (HR, 1.10; 95% CI, 0.88-1.36), compared to the age group of 65+. Female patients had a significantly higher risk of CSU compared to male ones (HR, 1.35; P=0.001). Patients with type 2 DM (P=0.871), hypertension (P=0.910), or dyslipidemia (P=0.696) did not have a significantly higher risk of CSU compared to those without. On the other hand, patients with allergic rhinitis (HR, 1.51; P<0.001), atopic dermatitis (HR, 2.44; P<0.001), or asthma (HR, 1.50; P<0.001) had a significantly higher risk of CSU compared to those without.
We found that patients with AITD had a greater risk of developing CSU compared to the control group after adjusting for demographic differences and comorbidities. In a study in Israel, 6 the diagnosis of CSU was associated with an increased odds ratio (OR) for hypothyroidism (OR, 17.338), hyperthyroidism (OR, 28.81), and anti-thyroid autoantibodies (P<0.001). However, the diagnosis was not specifically defined as Grave's disease and Hashimoto's thyroiditis.6 In a study in Italy,7 a history of autoimmune thyroiditis did not show a significantly increased risk of CSU. Only 34 CSU patients with a history of autoimmune thyroiditis were included.7 Other studies were hospital-based design with small sample size.101112131415 In our study, we obtained data from patients with the AITD and controls, and these methods were more appropriate for evaluating the association between AITD and CSU. Patients with Hashimoto's thyroiditis had a higher risk of developing CSU than those with Grave's disease, being consistent with the results of previous studies.1011 The exact mechanism linking AITD with CSU remains unknown. Previous reports have demonstrated that anti-thyroid autoantibodies are significantly increased in CSU patients compared to healthy patients.1216 Mozena et al.1 showed that neither anti-thyroglobulin nor anti-thyroid peroxidase (TPO) autoantibodies are capable of inducing activation of mast cells. Previous studies suggested that IgG antithyroid antibody rather than IgE antithyroid antibody is prevalent in the majority of patients with CSU. However, recent studies1718 suggested that specific IgE to TPO plays a role in the pathogenesis of CSU. Further studies are required to investigate possible interactions between anti-thyroid antibodies and CSU.
In our study, we found that the proportion of metabolic and allergic diseases was significantly higher in the AITD group compared to the control group. The association between AITD and type 1 type 2 DM was reported.1920 Evolving evidence of interactions between thyroid hormone and basal mechanisms controlling appetite and energy expenditure, as well as between the regulation of insulin sensitivity and secretion, explains the possible association between AITD and metabolic diseases.21 Previous studies reported a higher incidence of AITD in patients with allergic disease, and the relationship between allergic and autoimmune diseases was elucidated as 2 potential outcomes of dysregulated immunity.222324
The possible association between metabolic diseases and CSU has recently been proposed and investigated.252627 Ye et al.27 found a positive relationship between metabolic syndrome and CSU in a hospital-based study of 131 patients. In addition, Chung et al.26 reported that dyslipidemia is a risk factor for CSU in a population-based study. In another population-based study, Chang et al.25 showed that CSU is a risk factor for hypertension. However, in our study, there is no significantly increased risk of CSU in patients with type 2 DM, hypertension, or dyslipidemia. Previous studies suggested that allergic diseases, such as asthma and allergic rhinitis, could be associated with CSU.2829 In our study, we found that allergic diseases, including allergic rhinitis, atopic dermatitis, and asthma, had a significantly higher risk of CSU. Further studies are needed to elucidate the association between metabolic or allergic diseases and CSU in each disease and matched-control groups.
Our study has some limitations. First, diagnosis of CSU, AITD, or other comorbidities was defined on the basis of KCD codes. Second by, there could be other confounding factors. Thirdly, we did not obtain information on over-the-counter medications or evaluate drug the adherence. Despite these limitations, the strengths of our study are population-based design using a nationwide database and generalizability.
In conclusion, after adjusting for demographic differences and comorbidities, we demonstrated that patients with AITD may have a significantly higher risk of CSU compared to those without.
ACKNOWLEDGMENTS
This study used National Health Insurance Service-National Sample Cohort (NHIS-NSC) data (NHIS-2016-2-062) made by the NHIS. This work was subsidized by a research grant from the Korean Dermatological Association in 2014.
YSK and YMP designed the study. KDH performed statistical analysis. All authors contributed substantially to the interpretation of data. The paper was written by YSK and was critically revised by other authors. All authors approved the final version of the manuscript before submission.
References
1. Mozena JD, Tiñana A, Negri J, Steinke JW, Borish L. Lack of a role for cross-reacting anti-thyroid antibodies in chronic idiopathic urticaria. J Invest Dermatol. 2010; 130:1860–1865. PMID: 20182447.

2. Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014; 69:868–887. PMID: 24785199.
3. Maurer M, Church MK, Gonçalo M, Sussman G, Sánchez-Borges M. Management and treatment of chronic urticaria (CU). J Eur Acad Dermatol Venereol. 2015; 29(Suppl 3):16–32.

4. Fine LM, Bernstein JA. Guideline of chronic urticaria beyond. Allergy Asthma Immunol Res. 2016; 8:396–403. PMID: 27334777.

6. Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012; 129:1307–1313. PMID: 22336078.

7. Lapi F, Cassano N, Pegoraro V, Cataldo N, Heiman F, Cricelli I, et al. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016; 174:996–1004. PMID: 26872037.

8. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. Forthcoming 2016.

9. Cherepanov D, Raimundo K, Chang E, Eagan M, Zazzali JL, Solari PG, et al. Validation of an ICD-9-based claims algorithm for identifying patients with chronic idiopathic/spontaneous urticaria. Ann Allergy Asthma Immunol. 2015; 114:393–398. PMID: 25771155.

10. Verneuil L, Leconte C, Ballet JJ, Coffin C, Laroche D, Izard JP, et al. Association between chronic urticaria and thyroid autoimmunity: a prospective study involving 99 patients. Dermatology. 2004; 208:98–103. PMID: 15056996.

11. Leznoff A, Sussman GL. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients. J Allergy Clin Immunol. 1989; 84:66–71. PMID: 2754146.

12. Díaz-Angulo S, López-Hoyos M, Muñoz Cacho P, Fernández M, López-Escobar M, Rodríguez F, et al. Prevalence of thyroid autoimmunity in spanish patients with chronic idiopathic urticaria: a case-control study involving 343 subjects. J Eur Acad Dermatol Venereol. 2016; 30:692–693. PMID: 25627749.

13. Aamir IS, Tauheed S, Majid F, Atif A. Frequency of autoimmune thyroid disease in chronic urticaria. J Coll Physicians Surg Pak. 2010; 20:158–161. PMID: 20392376.
14. Najib U, Bajwa ZH, Ostro MG, Sheikh J. A retrospective review of clinical presentation, thyroid autoimmunity, laboratory characteristics, and therapies used in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2009; 103:496–501. PMID: 20084843.

15. Gangemi S, Saitta S, Lombardo G, Patafi M, Benvenga S. Serum thyroid autoantibodies in patients with idiopathic either acute or chronic urticaria. J Endocrinol Invest. 2009; 32:107–110. PMID: 19411805.

16. Cebeci F, Tanrikut A, Topcu E, Onsun N, Kurtulmus N, Uras AR. Association between chronic urticaria and thyroid autoimmunity. Eur J Dermatol. 2006; 16:402–405. PMID: 16935798.
17. Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase--a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011; 6:e14794. PMID: 21532759.
18. Shin YS, Suh DH, Yang EM, Ye YM, Park HS. Serum specific IgE to thyroid peroxidase activates basophils in aspirin intolerant urticaria. J Korean Med Sci. 2015; 30:705–709. PMID: 26028921.

19. Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract. 2010; 64:1130–1139. PMID: 20642711.

20. Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med. 1995; 12:622–627. PMID: 7554786.

21. Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol (Oxf). 2011; 75:1–9. PMID: 21521298.

22. D'Angelo G, Marseglia L, Manti S, Colavita L, Cuppari C, Impellizzeri P, et al. Atopy and autoimmune thyroid diseases: melatonin can be useful? Ital J Pediatr. 2016; 42:95. PMID: 27814774.
23. Pedullà M, Miraglia Del Giudice M, Fierro V, Arrigo T, Gitto E, Salpietro A, et al. Atopy as a risk factor for thyroid autoimmunity in children. J Biol Regul Homeost Agents. 2012; 26:S9–S14. PMID: 22691261.
24. Pedullá M, Fierro V, Papacciuolo V, Alfano R, Ruocco E. Atopy as a risk factor for thyroid autoimmunity in children affected with atopic dermatitis. J Eur Acad Dermatol Venereol. 2014; 28:1057–1060. PMID: 24118567.

25. Chang HW, Cheng HM, Yen HR, Hsu CY, Lee YC, Chiang JH, et al. Association between chronic idiopathic urticaria and hypertension: a population-based retrospective cohort study. Ann Allergy Asthma Immunol. 2016; 116:554–558. PMID: 27264565.
26. Chung SD, Wang KH, Tsai MC, Lin HC, Chen CH. Hyperlipidemia is associated with chronic urticaria: a population-based study. PLoS One. 2016; 11:e0150304. PMID: 26964045.

27. Ye YM, Jin HJ, Hwang EK, Nam YH, Kim JH, Shin YS, et al. Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013; 93:156–160. PMID: 22948845.

28. Isik SR, Karakaya G, Celikel S, Demir AU, Kalyoncu AF. Association between asthma, rhinitis and NSAID hypersensitivity in chronic urticaria patients and prevalence rates. Int Arch Allergy Immunol. 2009; 150:299–306. PMID: 19494528.

29. Nettis E, Pannofino A, D'Aprile C, Ferrannini A, Tursi A. Clinical and aetiological aspects in urticaria and angio-oedema. Br J Dermatol. 2003; 148:501–506. PMID: 12653742.

Table 1
Characteristics of the study patients of the AITD (n=3,659) and control group (n=18,295)
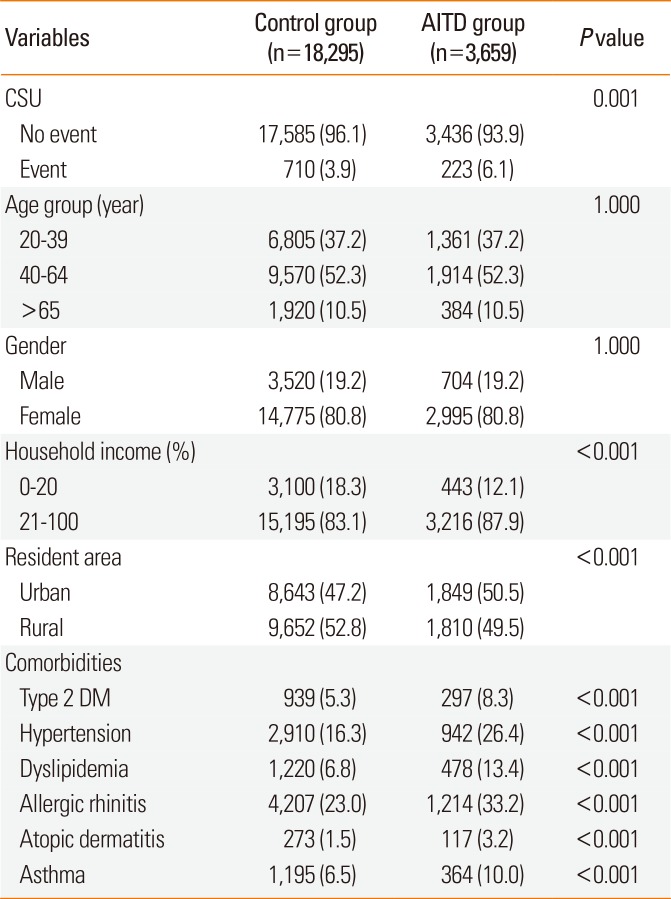
Table 2
Univariate and multivariate Cox regression analysis of CSU development during a 8-year follow-up period




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download