Abstract
Purpose
Because the true prevalence of food allergy (FA), as based on the results of an oral food challenge test (OFC), is unknown, it is likely that children with suspected FA unnecessarily eliminate potentially causative foods. This study aimed to identify the prevalence of FA and to determine the proportion of children who unnecessarily eliminate food.
Methods
To identify children with FA, a primary survey was conducted via a questionnaire with all children aged 0-18 years in Niijima village (remote islands of Japan). In the secondary survey, a detailed medical interview was conducted by doctors with children who currently did not eat some foods. The third survey involved serum food-specific immunoglobulin E (IgE) tests and an OFC for children with suspected FA.
Results
Of 376 enrolled children, 374 (99.5%) completed the questionnaire. Some foods were eliminated by 18.6% and 13.0% of all children and those ≥6 years old, respectively. The target population for the secondary survey included 69 children who all completed the medical interview. The target population for the third survey consisted of 35 children, of whom 26 (74.3%) underwent the blood test. An OFC was performed 35 times with 20 children. As a result, the prevalence of FA was 4.9% in children of all ages and 4.7% in those ≥6 years old. Moreover, 55.0% children were able to cease eliminating food intake.
An increasing prevalence of food allergy (FA) has recently been reported among children.12 However, the true prevalence of FA, as based on an oral food challenge test (OFC), is relatively unknown.3 Rona et al.4 reported that many studies of the prevalence of FA have been based only on perception of food reactions, and compared with studies that include objective diagnostic tools, most of these studies seem to have overestimated the prevalence of FA. The first author of this article, who worked as a physician in Niijima village from 2014 to 2016, was under the impression that food might be unnecessarily avoided by children. Niijima village, which consists of uninhabited islands and 2 inhabited islands named Niijima and Shikinejima, respectively, is located 160 km south of mainland Japan and accessed primarily by a daily ship or airplane. Because essential food items are transported from the mainland to the islands by ship, the foods and daily eating habits are similar to those of the mainland. Of the approximately 3,000 people living in Niijima village, 12.9% are younger than 18 years old. Because the movement of the population on these islands is relatively limited, the authors believed that identification of the true prevalence of FA, based on a census survey, was possible. Therefore, this study aimed to identify the prevalence of FA and to determine the proportion of children who unnecessarily eliminate food in Niijima village.
To determine patients with suspected FA, a primary survey was conducted via a questionnaire among all children aged 0-18 years (high school students and younger) in Niijima village. The distribution, response, and collection of the questionnaires occurred in July 2014 at each institution or by mail for children younger than preschool age. The parents completed the questionnaire; when the parents might not have been aware of their child's FA symptoms, the child was consulted.
The questionnaire collected information regarding the number of older siblings, length of time living in the islands, history of exclusive breast-feeding, history of infantile eczema, history of other allergic diseases, and family history of allergic diseases. The primary questions included those regarding the history of FA, foods to which there were current reactions, and foods currently avoided. Children with positive responses regarding current reactions and avoiding foods proceeded to the secondary survey.
The secondary and third surveys were conducted between August 2014 and February 2015. The secondary survey consisted of a detailed medical interview by doctors located on the islands. All kinds of foods which were suspicious for FA or had not been eaten yet were evaluated in children who were ≥6 years old as of the end of the year. For children who were ≤5 years old as of the end of the year, only 3 major food allergens (eggs, milk, and wheat) and foods for which there was a previous history of FA were evaluated. Allergy to peanuts was not evaluated in children aged ≤5 years because individuals in Japan and East Asia do not have a habit of eating peanuts daily, and the prevalence of peanut allergy in Japan was not as high as that in USA or Europe.156 The differences by age were based on the fact that children enter elementary school at 6 years old, and schools do not restrict food; therefore, all foods were tested for those children. In addition, diagnoses were based on results from previous tests of serum specific immunoglobulin E (IgE) antibodies for the suspected foods when available.
Oral allergy syndrome (OAS) was diagnosed as FA by confirming the symptoms several times through a medical interview. An obvious history of FA symptoms was also diagnosed as FA by a medical interview only. Children who could not be diagnosed with FA based on the interview proceeded to the third survey.
The third survey involved blood tests and an OFC when both children and their parents provided informed consent. Serum specific IgE antibodies for suspected foods were evaluated using the blood tests in children who had not previously undergone these tests within 1 year. In the OFC, the total amount that was eaten was defined according to each institution. For children who were ≥6 years old as of the end of the year, 40 g of eggs, 200 mL of milk, and 200 g of wheat were used. For children who were ≤5 years old as of the end of the year, 20 g of egg, 130 mL of milk, and 100 g of wheat were used. For other foods, the amount was determined based on the serving size provided by each institution. The form, ingested amount, and protein content of the food in the OFC are listed in Table 1.
The high-risk group was defined as children who had a previous history of anaphylaxis to the food and food-specific IgE antibody >3.5 kU/L (class 3). The low-risk group ate the full amount of the food at a time, while the amount was split into 2 or 3 unequal portions for the high-risk group. Furthermore, the total amount of the food was reduced for the high-risk group if the doctors judged that it was too risky to eat the full amount. FA symptoms were graded from 1 to 5 using the Sampson classification.7 Finally, the number of children who required food avoidance at the institution was compared between July 2014 and July 2015.
Diagnostic criteria for FA were a history of obvious FA symptoms (greater than grade 2) within the past year, OAS, specific IgE antibody >100 kU/L, or a positive OFC result, which was defined as appearance of grade 2 symptoms or as grade 1 symptoms lasting >30 minutes. Children with grade 1 symptoms that improved within 30 minutes were diagnosed with indeterminate FA and evaluated again at home within approximately 1 month to determine if the same symptoms occurred. After the OFC, children were advised whether they could eat the causative food.
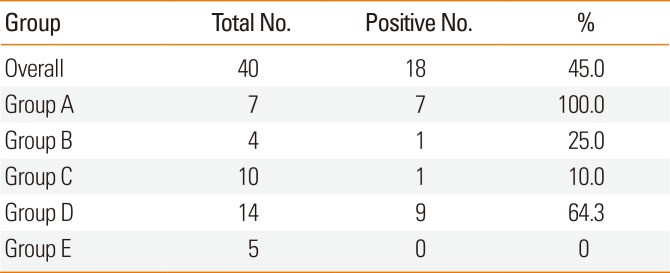
Based on history and food elimination, children who were divided into 5 groups (A to E) (see Fig. 1). Children in group A had experienced obvious FA symptoms (greater than grade 2 symptoms) within the previous year (or 0.5 year for children aged 0-3 years) and were currently completely eliminating causative foods. Children in group B had experienced FA symptoms more than 1 year prior (or 0.5 year for children aged 0-3 years) and were currently completely eliminating causative foods. Children in group C had not previously experienced FA symptoms but had never eaten potentially causative foods because of other reasons, such as positive specific IgE antibodies on a blood test or a sibling history of FA. Children in group D ate small amounts of potentially causative foods but still restricted their intake. Children in group E were able to eat without restriction.
The study was designed in accordance with the Declaration of Helsinki and guidelines for the ethics of an epidemiology investigation from the Ministry of Health, Labour, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology in Japan. We obtained written informed consent from all the patients and their parents included in this study as well as approval from the Ethics Committee of Tokyo National Health Insurance clinic in Niijima (Niijima-2014-001). The OFC was conducted in accordance with FA guidelines.89
Categorical variables are reported using frequency distributions and percentages, and continuous variables are reported using mean and standard deviation. Associations between categorical variables were evaluated using χ2 tests. A 2-sided significance level was set at 0.05. All statistical analyses were performed using SPSS version 22.0.0 (IBM Corp., Armonk, NY, USA).
The target population consisted of 376 children, of whom 374 (99.5%) completed the questionnaire (184 [49.3%] boys and 189 [50.7%] girls). Background characteristics are shown in Table 2. The sex could not be determined in 1 child. Of these children, 16% responded poorly to food in the past and 18.4% currently did not eat some foods. Thirteen percent of children ≥6 years old currently did not eat some foods.
The target population for the secondary survey included 69 children, who all completed the medical interview. Of these children, 20 were too young to consume some foods and were therefore regarded as FA negative. Nine children were diagnosed with FA by a medical interview only, including 6 children with OAS. On the other hand, 5 children confirmed a pattern of unnecessarily avoiding the causative food by a medical interview only. Children with FA negative results showed 2 patterns. The target population for the third survey included 35 children, of whom 26 (74.3%) underwent the blood test. Of the 9 children who withdrew, 7 (77.8%) were ≥6 years old, 5 did not want to eat the foods, 3 had been instructed by other doctors not to eat the foods, and 1 could not obtain the food within the study period. The OFC was performed 35 times on 20 children, resulting in a prevalence of FA of 4.9% for all ages and 4.7% for children ≥6 years old. The results of FA-positive children, including 8 children with positive OFC results, are listed in Table 3. One child was diagnosed with indeterminate FA and evaluated again at home; he was then found to have negative results for FA. The results obtained from each age group are listed in Table 4. A certain number of children were suspected with FA in each age group. Allergy to a hen's egg was observed in all age groups, and allergy to fruit was most frequent after school-going age.
The prevalences of FA with and without OAS were 3.6% and 2.7%, respectively. Food elimination was no longer necessary for 55.0% (22/40) of the children. The list of foods that were unnecessarily avoided is shown in Fig. 2. The overall study design and outcomes are summarized in Fig. 3.
The comparisons of question responses between the FA-positive and FA-negative participants are shown in Table 5. Significantly more FA-positive participants had a previous history of infantile eczema (P<0.001), other allergic diseases (P=0.001), and family history of allergy (P=0.012). According to the School Life Guidance and Management form, which must be submitted by parents of children with allergic diseases in Japan, the number of children who required food elimination at their institutions decreased from 16 in July 2014 to 8 in July 2015.
In subgroup analysis, group A included 7 children, group B included 4 children, group C included 10 children, group D included 14 children, and group E included 5 children. Regarding the groups, only 25.0% of the children in group B had FA, and only 10.0% of the children in group C had FA (Table 6).
In this survey, 55.0% of children with suspected FA were able to cease avoiding causative foods, indicating that a considerable number of children may unnecessarily eliminate food. Furthermore, only 25.0% of children in group B, who were eliminating foods despite not experiencing symptoms for more than 1 year, and 10.0% of children in group C, who eliminated foods without experiencing symptoms, were diagnosed with FA. Regarding the type of food, ingredients such as egg and milk, which are the main food allergen in Japan, were especially unnecessarily avoided. In addition, the primary food allergen for children older than school age in Japan, including crustaceans, fish eggs, and yams, were unnecessarily eliminated by children ≥6 years old. Therefore, the prevalence of FA to these ingredients might have been overestimated, particularly given the self-reported answers to the questionnaire. Although some children eliminated causative foods because of positive blood test results, a correct diagnosis based on an OFC could be very important. Resolving the need for elimination could help children enjoy eating, reduce parental anxiety regarding their children mistakenly eating causative foods, and reduce the need for institutions to manage specific lunch.
Regarding FA-related practices, it is important to determine if children can eat specific food at an earlier age. Of the 9 children who withdrew, 77.8% were ≥6 years old, and the most common reason for withdrawal was that they did not want to eat the food. As children grow older, they become more unwilling to eat potentially causative foods; therefore, it is difficult to determine whether they have FA or just dislike the foods. A recent study demonstrated that early intake of causative foods within safe limits, as based on OFC results, leads to early induction of tolerance to the food.101112 In addition, the present results suggested that early eating based on OFC results reduces the unwillingness to undergo an OFC. On the other hand, 3 children had been instructed by other doctors not to eat the causative foods. Therefore, challenges regarding the pathophysiology and appropriate treatment of FA remain, and doctors who do not specialize in FA need to be educated regarding the appropriate management of FA.
If safety criteria are established, it is preferable that low-risk children undergo an OFC in non-specialized facilities. The use of an OFC is currently recommended only for specialized hospitals.913 As a result, high-risk children might have long waiting periods to undergo an OFC because low-risk children also have to undergo OFCs at these facilities. In this study, safe OFCs could be conducted on the basis of standard OFC protocols. To achieve this, safety criteria to identify the low-risk children are required.
A past history of infantile eczema, other allergic diseases, and a family history of allergic diseases were significantly more common in participants with FA. Although being the first child was not significant, it tended to be more common in children with FA. These results are consistent with those of a previous report.11415
Two limitations of the present study might have resulted in underestimation of the prevalence of FA. First, the 9 children who withdrew had suspected FA. Secondly, because all foods were not evaluated in children aged ≤5 years, it is possible that some FA were not identified. In contrast, the present study may have overestimated the prevalence of FA because specific IgE antibody >100 kU/L was defined as FA without OFC.
In conclusion, we were able to determine a correct diagnosis based on OFC results. The prevalence of FA was 4.9%, and 55.0% of children with suspected FA were able to cease food elimination, indicating that a considerable number of children were unnecessarily eliminating food.
ACKNOWLEDGMENTS
We would like to express our deep appreciation to persons enrolled in the survey and the Niijima village public office, particularly the village mayor Hiroshi Ozawa for his cooperation. We also would like to thank the Clinical Research Support Team (CRST)-Jichi for their advice.
References
1. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014; 133:291–307. PMID: 24388012.

2. Carrard A, Rizzuti D, Sokollik C. Update on food allergy. Allergy. 2015; 70:1511–1520. PMID: 26443043.

3. Fleischer DM, Bock SA, Spears GC, Wilson CG, Miyazawa NK, Gleason MC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011; 158:578–583.e1. PMID: 21030035.

4. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007; 120:638–646. PMID: 17628647.

5. Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014; 69:992–1007. PMID: 24816523.

6. Akiyama H, Imai T, Ebisawa M. Japan food allergen labeling regulation--history and evaluation. Adv Food Nutr Res. 2011; 62:139–171. PMID: 21504823.

7. Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003; 111:1601–1608. PMID: 12777599.

8. NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010; 126:S1–S58. PMID: 21134576.
9. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS, et al. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009; 123:S365–S383. PMID: 19500710.
10. Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012; 130:473–480.e1. PMID: 22846751.

11. Kim JS, Nowak-Węgrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011; 128:125–131.e2. PMID: 21601913.

12. Sato S, Yanagida N, Ogura K, Imai T, Utsunomiya T, Iikura K, et al. Clinical studies in oral allergen-specific immunotherapy: differences among allergens. Int Arch Allergy Immunol. 2014; 164:1–9. PMID: 24943470.

13. Rancé F, Deschildre A, Villard-Truc F, Gomez SA, Paty E, Santos C, et al. Oral food challenge in children: an expert review. Eur Ann Allergy Clin Immunol. 2009; 41:35–49. PMID: 19585859.
14. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011; 127:594–602. PMID: 21236480.

15. Krogulska A, Dynowski J, Funkowicz M, Małachowska B, Wąsowska-Królikowska K. Prevalence and clinical impact of IgE-mediated food allergy in school children with asthma: a double-blind placebo-controlled food challenge study. Allergy Asthma Immunol Res. 2015; 7:547–556. PMID: 26333701.

Fig. 1
Subgroup analysis. Groups A and B had experienced obvious FA symptoms (group A was within the previous year), and were currently completely eliminating the causative foods. Group C had not experienced FA symptoms previously but had never eaten potential causative foods. Group D still restricted their intake. Group E were able to eat without restriction. FA, food allergy.
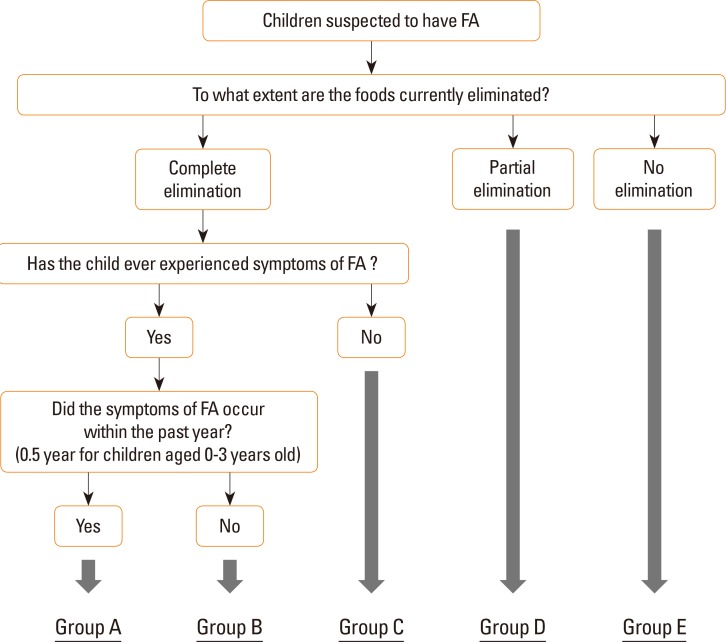
Fig. 2
List of foods that were unnecessarily avoided. Eggs and milk were the particular food types that were unnecessarily avoided by all ages. For children ≥6 years old, yams, crustaceans, and fish eggs were the particular food types that were unnecessarily avoided.
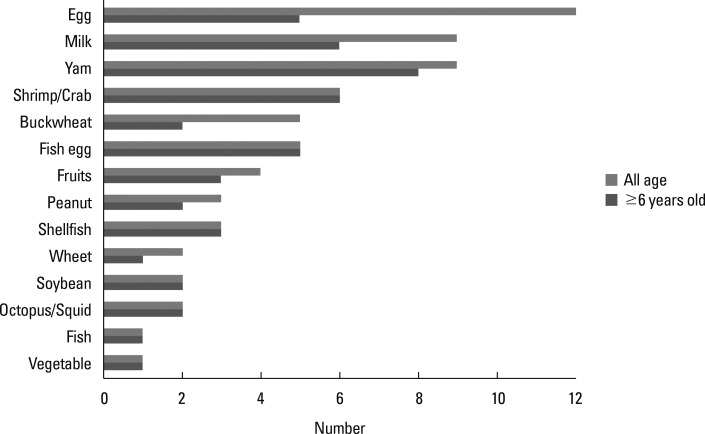
Fig. 3
Study design and clinical outcomes regarding FA based on the 3 surveys conducted on children in Niijima village. As a result, the prevalence of FA for all ages was 4.9% (18 children). FA, food allergy; OFC, oral food challenge.
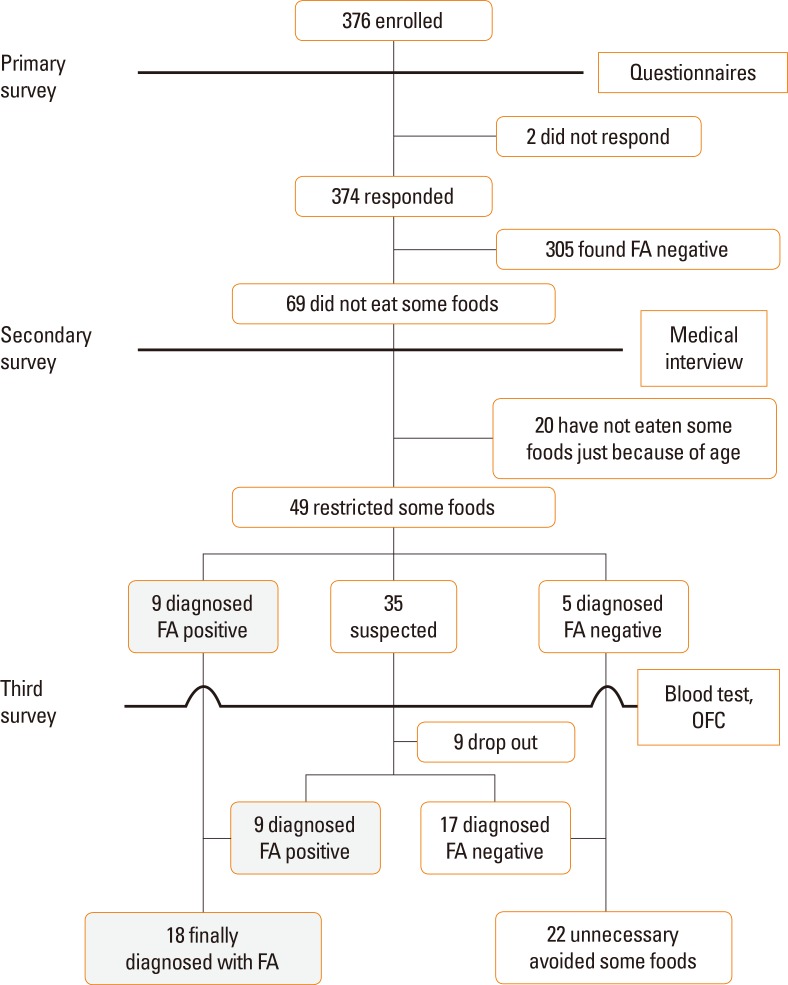
Table 1
The form, ingested amount, and protein content of foods in the OFC
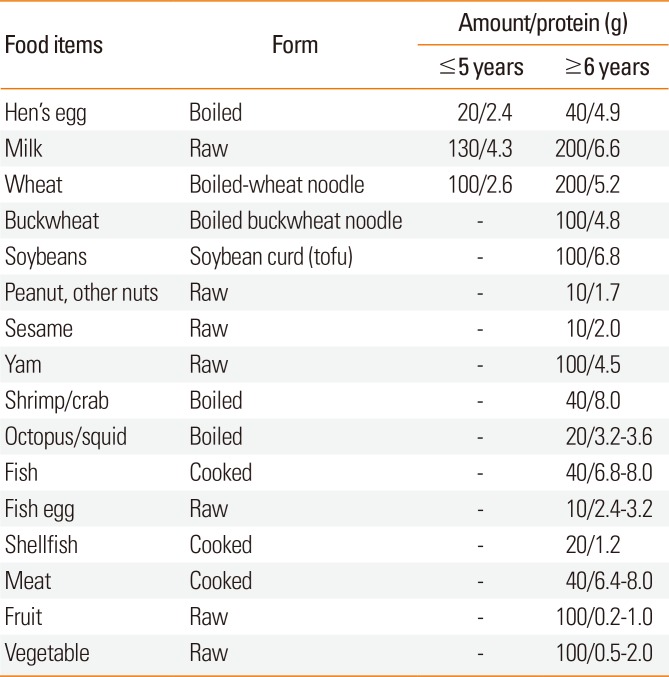
Table 2
Background characteristics of the 374 participants
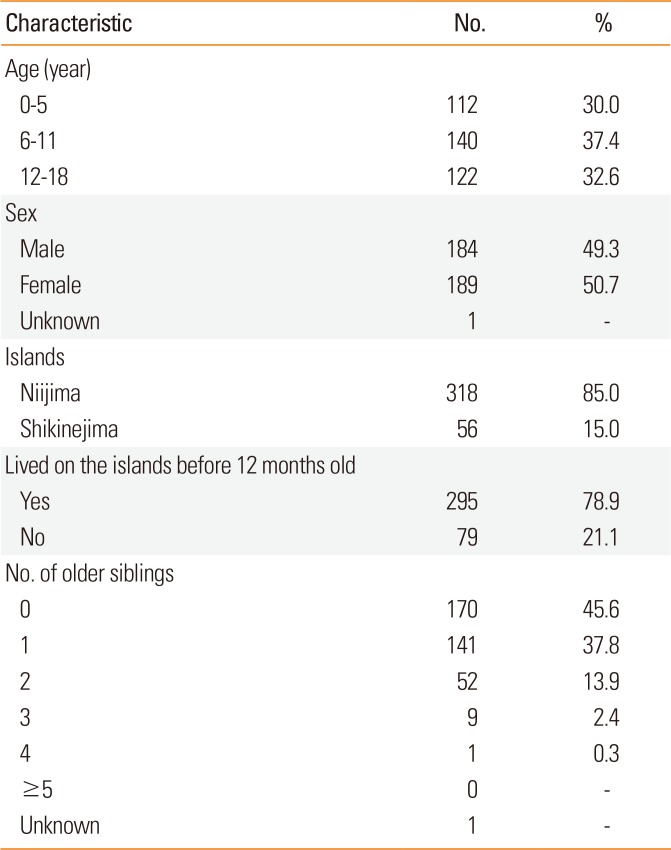
Table 3
List of children with FA
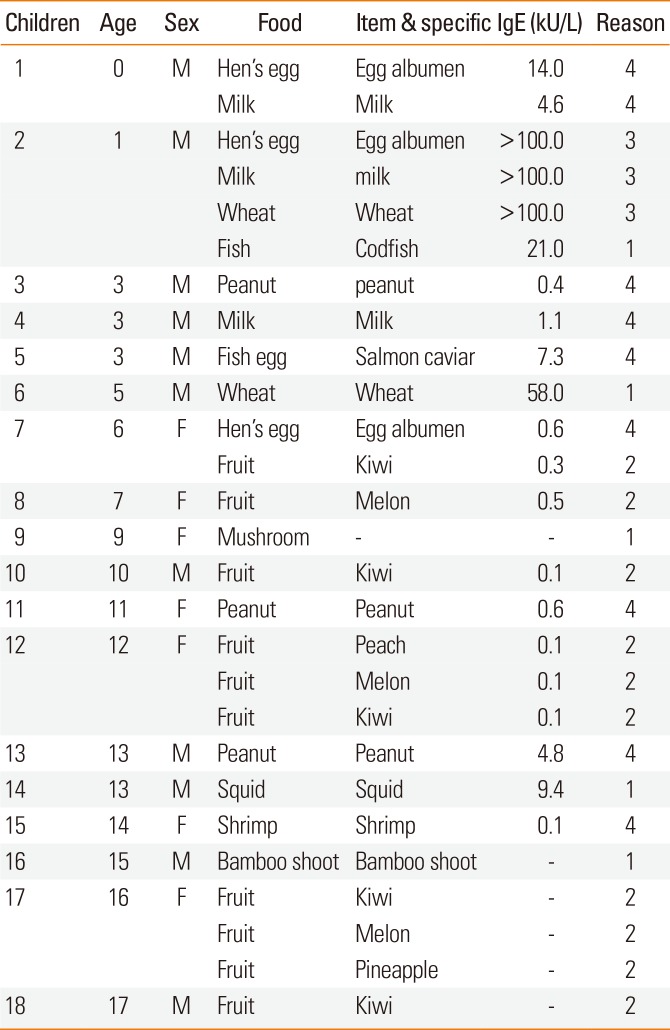
Table 4
Comparison of FA results obtained from each age group
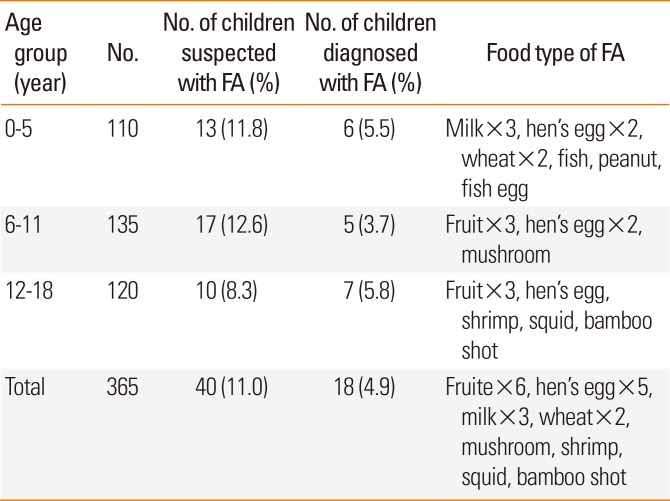
Table 5
Comparison of factors for FA in 374 children in the Niijima Islands




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download