Abstract
Chronic rhinosinusitis (CRS) is a heterogeneous inflammatory disease with various underlying pathophysiologic mechanisms which translate to endotypes, in contrast to clinical phenotypes or histological subtypes. Defining endotypes can help clinicians predict disease prognosis, select subjects suitable for a specific therapy, and assess risks for comorbid conditions, including asthma. Therefore, with recent advancement of biologicals in CRS clinical trials, endotyping can be a breakthrough in treating recalcitrant CRS. CRS is caused by dysregulated immunologic responses to external stimuli, which induce various inflammatory mediators from inflammatory cells, including innate lymphoid cells (ILCs) and T lymphocytes as well as epithelial cells. Thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33, which are mainly secreted by epithelial cells in response to external stimuli, act on type 2 ILCs and T helper 2 (Th2) cells, inducing IL-4, IL-5, and IL-13. Local immunoglobulin E (IgE) production is also a signature event in nasal polyps (NP). These inflammatory mediators are novel potential therapeutic targets for recalcitrant CRS. This article reviews recent publications regarding endotypes and endotype-based therapeutic strategies in CRS and NP.
Chronic rhinosinusitis (CRS) is one of the common diseases affecting about 10% of the general population, causing significant socioeconomic burden.12 The phenotypes of CRS, clinically observable characteristics, have been classified based on endoscopic or computed tomographic findings of nasal polyps (NP),3 the presence of lower airway disease (e.g. aspirin-exacerbated respiratory disease), and hypersensitivity to allergens (e.g. allergic fungal rhinosinusitis). Furthermore, CRS can also be subdivided by underlying diseases, such as cystic fibrosis, ciliary dyskinesia, autoimmune diseases, immune deficiency, and systemic illness.456 Specifically, NP are presented as a severe form of inflammation and remodeling among the phenotypes of CRS, and the presence of NP has been considered the most important clinical aspect of phenotyping CRS due to therapeutic challenges. For this reason, histologic subtypes of NP have been sought to be defined more precisely: eosinophilic or non-eosinophilic NP. Mucosal eosinophilia predicts recurrence after surgeries and risks of having comorbidities.7 However, there are no standard methods to evaluate tissue eosinophilia due to uneven distribution throughout the tissue.8 Multiple inflammatory cells exist in most NP tissue, but the function and pathological roles of these cells are not yet completely elucidated.9 Even in eosinophilic NP, neutrophils and macrophages exist and may play a role in nasal polypogenesis.2 Therefore, the subtyping of CRS or NP based on inflammatory cells has limitations. One phenotype can include several molecular mechanisms (endotypes), and one endotype can also involve several phenotypes. Due to these challenges, it is hard to design a personalized treatment approach with clinical phenotyping or histologic subtyping alone.
The concept of personalized medicine in the field of CRS can be incorporated after establishing endotypes. This is because CRS involves various pathomechanisms showing high heterogeneity, which causes different therapeutic responses. Endotyping also helps determine optimal primary therapeutic modality, select a good responder to a specific treatment, and predict treatment outcomes and risks for comorbidities, such as asthma.
We herein summarize pathophysiology and potential endotypes of CRS with NP (CRSwNP) and introduce biologicals available now or under investigation in CRS.
CRS can be understood as a dysfunctional host-environment interaction at the nasal and sinus mucosa.1011 Bacterial and viral infections, fungal extracts, and protease allergens play potential roles in CRS as external stimuli.12 For the evidence of bacterial roles, the superantigen hypothesis has been strongly suggested. Colonized Staphylococcus aureus secretes enterotoxins that augment local T helper 2 (Th2) inflammation and may play a role in the pathogenesis of NP as a disease modifier rather than an initiator.13 Another hypothesis of bacterial roles is that bacterial biofilms may be involved in CRS pathogenesis. Biofilms are organized structures comprised of bacteria protectively encased within extracellular matrix (ECM), which is associated with local inflammatory markers and disease severity.14 Although many studies have attempted to find evidence, the role of fungi in CRS has drawn lots of controversies in the last decade.15 There is still relatively sparse evidence that viral infection is a chronic source of CRS or involved in the initiation and development of sinonasal allergic inflammation. However, ds-RNA, a toll-like receptor 3 (TLR3) ligand, is a potent inducer of thymic stromal lymphopoietin (TSLP)16 and a potential changer of innate lymphoid cell (ILC) phenotypes,17 which may be associated with chronicity of airway disease. Moreover, viral infection has been clearly implicated in the exacerbation of allergic airway diseases, such as asthma, in a number of studies.18
The epithelial barrier is the first line of defense; its breakdown can play a significant role in allowing external stimuli to enter nasal tissue and provoke immune responses.19 Functional and mechanical defects have been reported in NP. The protease-activated receptor (PAR) contributes to the production of cytokines and chemokines from the epithelium in response to external stimuli, such as bacteria, fungi, and allergens.1220 Epithelial barrier destroyed by protease activities enables allergens to pass physical epithelial barriers, culminating in allergen sensitization.21 It also signals epithelial cells to secrete innate cytokines and then facilitates the induction of eosinophilic inflammation. Epithelial-derived innate cytokines, such as interleukin (IL)-25, IL-33, and TSLP, may also participate in the evolution of NP.22 IL-33 is secreted by immune cells, such as macrophages and dendritic cells as well as epithelial cells.23 Full-length IL-33 is extracellularly released when epithelial cells undergo necrosis and necroptosis via tissue damage caused by external stimuli. Biologically active full-length IL-33 plays a role in mucosal inflammation recruiting neutrophils via chemokines, including chemokine (C-X-C motif) ligand (CXCL)-1 and CXCL-2.242526 Gordon et al.27 have suggested a potential role of IL-33 in eosinophilic inflammation by demonstrating a splice variant of IL-33 missing exons 3 and 4, which localizes to the cytoplasm of epithelial cells, is actively released and strongly related to Th2 inflammation, whereas full length is not. Several studies have sought to investigate the expression and role of IL-33 in CRS. There have been conflicting results of the expression of IL-33 in CRS. It has been reported that IL-33 mRNA is highly expressed in nasal mucosa but was not elevated in NP or other inflamed areas of the sinuses in CRSwNP.282930 A significant up-regulation of ST2 expression has been demonstrated in ethmoid mucosa from CRSwNP, but the concentration of IL-33 protein is not significantly different between nasal polyp and control tissue.31 Kim et al.23 have recently demonstrated that IL-33 is up-regulated in other CRS tissues compared to eosinophilic NP and correlates with Th1/Th17 cytokines. IL-33 may contribute to the induction of different types of inflammation under various microenvironments.
IL-17E, also known as IL-25, is released by Th2 cells, mast cells, eosinophils as well as epithelial cells. It is produced and stored in the cytoplasm of epithelial cells as a result of external stimuli, including allergen proteases.32 IL-25 transcript levels have been reported to increase in CRS tissues, including NP and to correlate with disease severity and blood eosinophilia,3033 whereas an earlier study has reported that IL-25 and GATA-binding protein 3 (GATA-3) transcripts were decreased in NP vs control tissues.29 Additionally, polyp-derived IL-17RB (+) Th2 cells were identified in NP, which co-expressed ST2 and enhanced IL-5 and IL-13 production in response to IL-25 and IL-33.34 Protein levels of IL-25 are up-regulated in non-eosinophilic NP as well as eosinophilic NP.2235 Of note, the fact that IL-25, known as a cytokine involved in diverse Th2-mediated diseases, also correlated with inflammatory mediators involved in Th1 and Th17 responses in Asian subjects suggests that it may play diverse roles in polypogenesis besides promoting Th2 inflammation.3336 Blockade of IL-25 reduced the burden of NP in a mouse model of NP and represented a potential novel therapeutic target.36
TSLP is well known to be induced in airway epithelial cells by viruses, TLR3 agonists, protease, and pro-inflammatory cytokines.16373839 IL-1β and tumor necrosis factor (TNF)-α regulate TSLP transcript expression in an nuclear factor-kappa B (NF-κB)-dependent manner.39 Several researchers have demonstrated that TSLP mRNA is overexpressed in eosinophilic NP and associated with Th2 inflammation.404142 TSLP induces the differentiation of naïve T cells into effector Th2 cells via enhancement of OX40 ligand (OX40L)-OX40 axis on the interaction between dendritic cells and CD4 T cells.43 TSLP protein is post-translationally modified by the endogenous protease. The cleaved TSLP shows higher activity, producing IL-5 when stimulated with IL-1β, than the full-length form.40 Of interest, Kim et al.44 authors recently demonstrated that TSLP production was induced by periostin in epithelial cells under the Th2 high inflammatory condition like eosinophilic NP. Until now, TSLP has been consistently reported to play a pathological role in eosinophilic NP unlike IL-25 and IL-33.
Epithelial-derived cytokines, such as IL-25, IL-33, and TSLP, exert effects on type 2 ILCs (ILC2s).45 ILCs are lymphocyte-like cells, but lack markers of mature lymphocytes, and do not express allergen-specific T cell receptors. ILC2s are regarded as innate counterparts of Th2 cells because both share the same functional module by their mutual production of signature cytokines, such as IL-5 and IL-13.46 For example, GATA-3 is a key transcriptional factor that plays parallel roles in the development and function of both Th2 cells and ILC2s.47 Moreover, the signal transducer and activator of transcription (STAT)-6 is also an important factor for Th2 polarization and plays a role in the post-developmental role in ILC2s, though it is not required for the development of ILC2s.46 Interestingly, IL-33- and IL-25-activated ILC2s can induce eosinophilic airway inflammation accompanied by airway hyperresponsiveness even in recombination-activating gene (Rag) knockout mice, which means ILC2s have function independent of acquired immunity.4849 ILC2s are abundant and also have a close relationship with higher tissue and blood eosinophilia in NP, clinically related to worsening nasal symptom scores and asthma comorbidity.5051 Bal et al.52 have reported that there is spatial co-localization between ILC2s and eosinophils in NP, and that co-culture of eosinophils and ILC2s augmented the activation of eosinophils and prolonged their survival, and in return pre-activated eosinophils enhanced IL-5 production of ILC2s in an IL-4 dependent manner. Of note, ILC2s have functional plasticity responsive to environmental cues, including viral infection. Mouse ILC2s in the lung undergo T-bet-mediated plasticity in response to infection, including influenza virus, respiratory syncytial virus, Haemophilus influenza, and Staphylococcal aureus.17 Human ILC2s can be converted into ILC1s by IL-12 and reversed by IL-4,52 or into interferon (IFN)-γ/IL-13 dual-producing ILC1s in response to both IL-1β and IL-12.53 T cells that are able to produce both IFN-γ and IL-13 induce enhanced airway hyperresponsiveness compared to conventional Th2 cells.54 Thus, ILC2 plasticity may contribute to disease heterogeneity which might lead to recalcitrance and exacerbation of inflammatory diseases.
Three major subsets of CD4+ T effectors, classified as Th1, Th2, and Th17, function in host defense in response to various types of pathogens and are involved in different types of tissue injury in immunologic diseases. Regulatory T cells (Tregs) modulate T cell response inducing the termination of inflammation. Definite characteristics of differentiated subsets of T effector cells are cytokines they produce and transcriptional factors they express. Signature cytokines produced by the major CD4+ T cell subsets are IFN-γ for Th1 cells; IL-4, IL-5, and IL-13 for Th2 cells; IL-17 and IL-22 for Th17 cells, and IL-10 and transforming growth factor-beta (TGF-β) for Treg. Involved transcriptional factors are T-bet for Th1, GATA-3 for Th2, retinoid-related orphan receptor C (RORC) for Th17, and FOXP3 for Treg. CRS was initially classified according to the presence of NP which reflects predominantly eosinophil-infiltrating Th2 inflammation at the molecular levels in Western countries.55 However, Asian populations, including Chinese population, showed multiple inflammatory cell infiltrates, including neutrophils and T cells classified as Th1/Th17 cells.56 Furthermore, mixed Th17/Th2 inflammatory patterns were also demonstrated throughout a single nasal polyp tissue, and eosinophilic NP also encompass neutrophils and their biological markers such as IL-8 and myeloperoxidase.2 Therefore, NP begin to be understood from the dichotomization of Th1 or Th2 to a multi-dimensionally evolved concept. A European multicenter case-control study group (GA2LEN Sinusitis Cohort group) recently conducted the clustering of CRS subjects by T cell subset-based cytokines and explored whether these cytokines adequately reflect phenotype, such as the presence of NP or asthma comorbidities.57 This study analyzed tissue cytokines from 173 CRS subjects and 89 controls in a phenotype-free manner and classified 10 clusters: 4 clusters with low or undetectable IL-5, immunoglobulin E (IgE), and eosinophilic cationic protein (ECP); and 6 clusters having moderate to high concentrations of these markers. In detail, cluster 1 has minimal inflammatory markers, cluster 2 associated with Th22, cluster 3 representing Th1, and cluster 4 showing mixed Th17/Th22/Th1. The latter 6 clusters with elevated IL-5 were classified into 2 groups: clusters 8-10 showed the highest IL-5 with enhanced IgE against Staphylococcal enterotoxins plus neutrophilic markers plus Th17/Th22 cytokines implying sophisticated pathomechanism, whereas clusters 5-7 showed mainly IL-5 dominant CRS. This clustering roughly reflects phenotypes: clusters 1-3 represent CRS without NP (CRSsNP) and low asthma comorbidity, but the proportion of NP and asthma comorbidities seems to increase from cluster 4 to cluster 10. Furthermore, it was reported to be a distinct regional difference in endotypes.2 CRSwNP tissues from patients of European countries, such as Benelux and Berlin, had a high proportion of IL-5 (62%-82%); those from Adelaide, Australia showed IL-5 in 46% and mixed IL-5 plus IL-17 or IFN-r in 27%. Most of the Western NP were Th2-biased. However, there was a remarkable difference even in Chinese NP. Beijing NP showed mixed IL-5/IL-17 portion of 42%; NP in Chengdu, a Chinese city, mainly had a negative cytokine pattern of 57%. These results imply that CRS is not a dichotomous disease but has multi-dimensional continuum worldwide. To make this concept clearer and more understandable, longitudinal cohort studies would be required rather than cross-sectional, case-control studies conducted in the past.
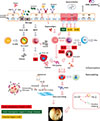
CRS is characterized by edematous remodeling patterns, basement membrane thickening, and goblet cell hyperplasia.5859 TGF-β is not only a chemoattractant for fibroblasts, enhancing their proliferation and collagen deposition, but also an inducer for Treg. High TGF-β is characteristic for CRSsNP, but low TGF-β was a signature finding in patients with CRSwNP.5860 Low TGF-β is also associated with a defect of Treg, namely, failing to maintain homeostasis, additionally, failure of deposition of collagens and other ECMs. High matrix metallopeptidases (MMPs), which are usually observed in NP, support ECM degradation.61 Besides the roles of TGF-β in the remodeling process in NP, the dysregulated coagulation system can be engaged in this process. Coagulation and fibrinolysis cascades are the biological processes involved in fibrin deposition and dissolution.62 The first study to suggest local activation of the coagulation system in sinus tissue showed that the concentration of thrombin and thrombin-antithrombin complex were increased in CRSwNP, which in turn participated in the remodeling process via a vascular endothelial growth factor pathway.63 Since that study, well-conducted reports have documented that the down-regulation of tissue plasminogen activator (t-PA) and activated coagulation factor XIIIa are involved in fibrin deposition and enhancement of fibrin crosslinking, culminating in edematous remodeling patterns in NP.636465 Of interest, the concentration of t-PA in CRS tissues shows a negative correlation with the concentration of ECP, a marker of eosinophils, and t-PA is mainly stained in epithelial cells on immunohistochemistry and down-regulated in epithelial cells by Th2 cytokines, such as IL-4 and IL-13.64 Furthermore, M2 macrophages, alternatively activated by Th2 cytokines,66 produce coagulation factor XIIIa which acts enzymatically and contribute to the formation of a tight tetrameric complex (FXIIIA2B2), a cross-linking process of fibrin.65 In summary, Th2-induced coagulopathy causes excessive fibrin deposition to induce edematous remodeling patterns in NP. The pathogenesis of NP described above is depicted in Figure.
Therapeutic strategies according to clinical or pathologic markers have also been developed. Among these signs, tissue eosinophilia is the most important regarding disease recurrence and comorbidities.7 Approximately 70%-80% of CRSwNP cases accounts for eosinophilic types in the Western country, whereas 30%-40% cases constitute eosinophilic NP in Asian countries, including South Korea and China.29 Eosinophilic CRS is a better responder to steroid therapy vs non-eosinophilic CRS.67 Non-eosinophilic CRS seems to be a better responder to surgical treatment vs eosinophilic CRS, especially in elderly subjects.68 Therefore, how to discriminate between 2 groups by using clinical features or easily available samples, such as blood samples, has been investigated because tissue eosinophil count using biopsy tissue is not practical for primary care clinicians. Blood eosinophilia, computerized tomography (CT) scans showing ethmoidal dominance, bilateral disease, asthma comorbidity, and aspirin sensitivity are predictors of eosinophilic NP.6970 Despite strategical approaches according to histologic or clinical subtypes, there must be mixed pathologic subgroups in recalcitrant subgroups which require further innovative therapeutic plans. That is why endotyping and the development of biologicals corresponding to their endotypes are inevitable at present. When we determine molecular targets from previous research data, Th2 cytokines, such as IL-4, IL-5, IL-13, and periostin, epithelial-derived cytokines, such as TSLP, IL-33, and IL-25, IgE as an acquired immunity marker, and Th17 cytokines such as IL-17a and IL-22, can be candidates for biologicals. Among these, several human monoclonal antibodies became commercially available or under clinical trials in allergic disease.
A humanized monoclonal anti-IgE antibody, omalizumab, which binds to free IgE with high affinity, has been used in moderate to severe allergic asthma in the US and Europe.71 Hanania et al.72 have demonstrated that periostin is a marker for omalizumab responsiveness in patients with moderate to severe allergic asthma. Since the concentration of IgE is locally increased in eosinophilic NP,73 a strategy for antagonizing IgE might be relevant in subjects with eosinophilic NP. However, a clear indication of omalizumab remains undetermined in NP.74 Given that periostin is upregulated and secreted from mast cells via IgE-mediated signaling in eosinophilic NP,44 periostin might be a biomarker for responsiveness to omalizumab in eosinophilic NP. Gevaert et al.75 conducted a randomized, double-blind, placebo-controlled study on the effects of omalizumab on NP and comorbid asthma (n=24). A significant decrease in the total nasal endoscopic polyp score after 16 weeks in the omalizumab-treated group was observed. Omalizumab also showed significant benefits in nasal and respiratory symptoms, such as nasal congestion, anterior rhinorrhea, loss of smelling sense, wheezing, and dyspnea, as well as quality-of-life scores, irrespective of the presence of allergy. However, there was no reduction in nasal or serum inflammatory mediators in the treated group. Larger sizes of clinical trials are needed to confirm this proof-of-concept study and to analyze subgroups for appropriate biomarkers to guide better treatment responses.
Eosinophils, mast cells, and ILC2s mainly produce IL-5 which is a strong driver to Th2 inflammation and associated with a higher risk of having asthma comorbidity.76 As mentioned above, 80% of Western NP cases account for the eosinophilic type. Furthermore, over 80% of European NP cases and 20%-60% of Asian NP cases expressed IL-5 in NP tissue homogenates.2 Based on previous mechanistic studies, antagonizing IL-5 would be considered a good therapeutic target. In a small clinical trial, subjects treated with anti-IL-5 mAb (reslizumab) showed reduced polyp size, blood eosinophilic counts, and ECP concentration in nasal secretions. Responders had higher IL-5 levels in nasal secretions compared with non-responders. However, there was no symptom improvement in the treated group.77 A clinical trial of another anti-IL-5 mAb, mepolizumab, demonstrated similar results as a previous study and showed a significant reduction in polyp score, blood eosinophilic count, serum ECP, and serum IL-5Rα, but no significant improvement in symptom scores.78 based on earlier studies, it still remains unclear which biomarker can be used to select good responders to anti-IL-5 treatment.
Dupilumab targeting IL-4Rα has inhibitory effects on the signaling of IL-4 and IL-13 since both cytokines signaled through IL-4Rα as a common subunit. IL-4 and IL-13 act through 2 different receptors. One is type 1, activated by IL-4 only and expressed on the lymphocyte. The other is type 2 receptor activated by both IL-4 and IL-13 and expressed by various cells.71 Blocking the type 2 receptor is promising for controlling recalcitrant allergic disease.7980 Since type 2 receptors can play a pivotal role in polypogenesis, dupulimab may play a role in treating recalcitrant NP. Recently, a multi-center clinical trial demonstrated a potential role of dupilumab in NP showing a significant decrease in nasal polyp score and improvements in olfaction and CT scores as well as other clinical outcomes, such as nasal symptoms and quality of life.81
Epithelial cell-derived innate cytokines, including TSLP, exert major effects on Th2 inflammation. However, there has been no study regarding its effect on NP, although a previous study investigated the inhibitory effects of anti-TSLP on allergen-induced asthmatic response.82 The roles of IL-25 and IL-33 in NP remain unclear and need further investigation.83 Therefore, targeting epithelial derived innate cytokines can be promising because it may control upstream mediators which T cell subsets do not act on.
A clinically observable phenotype includes multiple molecular endotypes with different prognoses. Therefore, phenotyping is not sufficient to predict responsiveness to medical or surgical treatments and the risk of comorbid conditions. With the advent of an era with biologicals, endotyping helps select patients suitable for each biological which can be a breakthrough in treating NP. Although modulating acquired immunity, for example T cell subsets, has made some progression, targeting epithelial cell-derived innate cytokines, such as TSLP, IL-33, and IL-25, may provide novel opportunities to manage CRS and NP as well as allergic airway disease.
Figures and Tables
ACKNOWLEDGMENTS
This research was supported by grant support from National Institutes of Health (NIH) 1K23AI110731 and the American Heart Association 11SDG7590063.
References
1. Kim DH, Han K, Kim SW. Effect of chronic rhinosinusitis with or without nasal polyp on quality of life in South Korea: 5th Korea National Health and Nutrition Examination Survey Korean. Clin Exp Otorhinolaryngol. 2016; 9:150–156.
2. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; 138:1344–1353.
3. Kim DW, Kim JY, Jeon SY. The status of the olfactory cleft may predict postoperative olfactory function in chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy. 2011; 25:e90–e94.
4. Cho SH, Bachert C, Lockey RF. Chronic rhinosinusitis phenotypes: an approach to better medical care for chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2016; 4:639–642.
5. Zhang L, Zhang L, Zhang CH, Fang XB, Huang ZX, Shi QY, et al. The lung function impairment in non-atopic patients with chronic rhinosinusitis and its correlation analysis. Clin Exp Otorhinolaryngol. 2016; 9:339–345.
6. Lee SH, Kim HJ, Lee JW, Yoon YH, Kim YM, Rha KS. Categorization and clinicopathological features of chronic rhinosinusitis with eosinophilic mucin in a Korean population. Clin Exp Otorhinolaryngol. 2015; 8:39–45.
7. Lou H, Meng Y, Piao Y, Wang C, Zhang L, Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015; 29:350–356.
8. Bachert C, Akdis CA. Phenotypes and emerging endotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2016; 4:621–628.
9. Kim DK, Park MH, Chang DY, Eun KM, Shin HW, Mo JH, et al. MBP-positive and CD11c-positive cells are associated with different phenotypes of Korean patients with non-asthmatic chronic rhinosinusitis. PLoS One. 2014; 9:e111352.
10. Kim DW, Eun KM, Jin HR, Cho SH, Kim DK. Prolonged allergen exposure is associated with increased thymic stromal lymphopoietin expression and Th2-skewing in mouse models of chronic rhinosinusitis. Laryngoscope. 2016; 126:E265–E272.
11. Cavone L, Cuppari C, Manti S, Grasso L, Arrigo T, Calamai L, et al. Increase in the level of proinflammatory cytokine HMGB1 in nasal fluids of patients with rhinitis and its sequestration by glycyrrhizin induces eosinophil cell death. Clin Exp Otorhinolaryngol. 2015; 8:123–128.
12. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012; 3 p preceding table of contents1–298.
13. Zhang N, Gevaert P, van Zele T, Perez-Novo C, Patou J, Holtappels G, et al. An update on the impact of Staphylococcus aureus enterotoxins in chronic sinusitis with nasal polyposis. Rhinology. 2005; 43:162–168.
14. Foreman A, Holtappels G, Psaltis AJ, Jervis-Bardy J, Field J, Wormald PJ, et al. Adaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Allergy. 2011; 66:1449–1456.
15. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.
16. Kato A, Favoreto S Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007; 179:1080–1087.
17. Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016; 17:626–635.
18. Hashimoto S, Matsumoto K, Gon Y, Ichiwata T, Takahashi N, Kobayashi T. Viral infection in asthma. Allergol Int. 2008; 57:21–31.
19. Zhang N, Van Crombruggen K, Gevaert E, Bachert C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy. 2016; 71:295–307.
20. Cho SH, Kim DW, Gevaert P. Chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol Pract. 2016; 4:575–582.
21. Cho SH, Kim DW, Lee SH, Kolliputi N, Hong SJ, Suh L, et al. Age-related increased prevalence of asthma and nasal polyps in chronic rhinosinusitis and its association with altered IL-6 trans-signaling. Am J Respir Cell Mol Biol. 2015; 53:601–606.
22. Liao B, Cao PP, Zeng M, Zhen Z, Wang H, Zhang YN, et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy. 2015; 70:1169–1180.
23. Kim DK, Jin HR, Eun KM, Mo JH, Cho SH, Oh S, et al. The role of interleukin-33 in chronic rhinosinusitis. Thorax. Forthcoming 2016.
24. Luzina IG, Pickering EM, Kopach P, Kang PH, Lockatell V, Todd NW, et al. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol. 2012; 189:403–410.
25. Mizutani N, Nabe T, Yoshino S. IL-17A promotes the exacerbation of IL-33-induced airway hyperresponsiveness by enhancing neutrophilic inflammation via CXCR2 signaling in mice. J Immunol. 2014; 192:1372–1384.
26. Lan F, Yuan B, Liu T, Luo X, Huang P, Liu Y, et al. Interleukin-33 facilitates neutrophil recruitment and bacterial clearance in S. aureus-caused peritonitis. Mol Immunol. 2016; 72:74–80.
27. Gordon ED, Simpson LJ, Rios CL, Ringel L, Lachowicz-Scroggins ME, Peters MC, et al. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci U S A. 2016; 113:8765–8770.
28. Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013; 188:432–439.
29. Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014; 69:1154–1161.
30. Lam M, Hull L, McLachlan R, Snidvongs K, Chin D, Pratt E, et al. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; 3:121–128.
31. Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014; 124:E115–E122.
32. Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015; 15:98–103.
33. Xu J, Han R, Kim DW, Mo JH, Jin Y, Rha KS, et al. Role of interleukin-10 on nasal polypogenesis in patients with chronic rhinosinusitis with nasal polyps. PLoS One. 2016; 11:e0161013.
34. Lam EP, Kariyawasam HH, Rana BM, Durham SR, McKenzie AN, Powell N, et al. IL-25/IL-33-responsive TH2 cells characterize nasal polyps with a default TH17 signature in nasal mucosa. J Allergy Clin Immunol. 2016; 137:1514–1524.
35. Iinuma T, Okamoto Y, Yamamoto H, Inamine-Sasaki A, Ohki Y, Sakurai T, et al. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2015; 114:289–298.
36. Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015; 135:1476–1485.e7.
37. Lee HC, Headley MB, Loo YM, Berlin A, Gale M Jr, Debley JS, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012; 130:1187–1196.e5.
38. Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009; 183:1427–1434.
39. Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007; 104:914–919.
40. Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013; 132:593–600.e12.
41. Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015; 64:121–130.
42. Boita M, Garzaro M, Raimondo L, Riva G, Mazibrada J, Pecorari G, et al. Eosinophilic inflammation of chronic rhinosinusitis with nasal polyps is related to OX40 ligand expression. Innate Immun. 2015; 21:167–174.
43. Liu T, Li TL, Zhao F, Xie C, Liu AM, Chen X, et al. Role of thymic stromal lymphopoietin in the pathogenesis of nasal polyposis. Am J Med Sci. 2011; 341:40–47.
44. Kim DW, Kulka M, Jo A, Eun KM, Arizmendi N, Tancowny BP, et al. Cross-talk between human mast cells and epithelial cells by IgEmediated periostin production in eosinophilic nasal polyps. J Allergy Clin Immunol. Forthcoming 2016.
45. Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12:1055–1062.
46. Robinette ML, Colonna M. Immune modules shared by innate lymphoid cells and T cells. J Allergy Clin Immunol. 2016; 138:1243–1251.
47. Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014; 40:378–388.
48. Morita H, Moro K, Koyasu S. Innate lymphoid cells in allergic and nonallergic inflammation. J Allergy Clin Immunol. 2016; 138:1253–1264.
49. Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012; 129:216–227.e1-6.
50. Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Cellular comparison of sinus mucosa vs polyp tissue from a single sinus cavity in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015; 5:14–27.
51. Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015; 45:394–403.
52. Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016; 17:636–645.
53. Ohne Y, Silver JS, Thompson-Snipes L, Collet MA, Blanck JP, Cantarel BL, et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016; 17:646–655.
54. Sugimoto T, Ishikawa Y, Yoshimoto T, Hayashi N, Fujimoto J, Nakanishi K. Interleukin 18 acts on memory T helper cells type 1 to induce airway inflammation and hyperresponsiveness in a naive host mouse. J Exp Med. 2004; 199:535–545.
55. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289.
56. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968.
57. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; 137:1449–1456.e4.
58. Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010; 125:1061–1068.
59. Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009; 124:253–259.e1-2.
60. Van Bruaene N, C PN, Van Crombruggen K, De Ruyck N, Holtappels G, Van Cauwenberge P, et al. Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy. 2012; 42:883–890.
61. Van Bruaene N, Bachert C. Tissue remodeling in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2011; 11:8–11.
62. Kim DY, Cho SH, Takabayashi T, Schleimer RP. Chronic rhinosinusitis and the coagulation system. Allergy Asthma Immunol Res. 2015; 7:421–430.
63. Shimizu S, Gabazza EC, Ogawa T, Tojima I, Hoshi E, Kouzaki H, et al. Role of thrombin in chronic rhinosinusitis-associated tissue remodeling. Am J Rhinol Allergy. 2011; 25:7–11.
64. Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013; 187:49–57.
65. Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013; 132:584–592.e4.
66. Krysko O, Holtappels G, Zhang N, Kubica M, Deswarte K, Derycke L, et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy. 2011; 66:396–403.
67. Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012; 129:1522–1528.e5.
68. Kim DW, Kim DK, Jo A, Jin HR, Eun KM, Mo JH, et al. Age-related decline of neutrophilic inflammation is associated with better postoperative prognosis in non-eosinophilic nasal polyps. PLoS One. 2016; 11:e0148442.
69. Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015; 70:995–1003.
70. Kim DK, Jin HR, Eun KM, Mutusamy S, Cho SH, Oh S, et al. Non-eosinophilic nasal polyps shows increased epithelial proliferation and localized disease pattern in the early stage. PLoS One. 2015; 10:e0139945.
71. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015; 136:1431–1440.
72. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811.
73. Baba S, Kondo K, Toma-Hirano M, Kanaya K, Suzukawa K, Ushio M, et al. Local increase in IgE and class switch recombination to IgE in nasal polyps in chronic rhinosinusitis. Clin Exp Allergy. 2014; 44:701–712.
74. Vennera Mdel C, Picado C, Mullol J, Alobid I, Bernal-Sprekelsen M. Efficacy of omalizumab in the treatment of nasal polyps. Thorax. 2011; 66:824–825.
75. Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013; 131:110–116.e1.
76. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to Staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; 126:962–968. 968.e1–968.e6.
77. Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006; 118:1133–1141.
78. Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011; 128:989–995.e1-e8.
79. Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014; 371:130–139.
80. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016; 388:3–4.
81. Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016; 315:469–479.
82. Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014; 370:2102–2110.
83. Lee M, Kim DW, Shin HW. Targeting IL-25 as a novel therapy in chronic rhinosinusitis with nasal polyps. Curr Opin Allergy Clin Immunol. 2017; 17:17–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download