Abstract
Lichenoid drug eruption (LDE) is a rare form of delayed-type drug eruption. Among anti-tuberculosis (Tb) agents, cycloserine (CS) has been reported as a rare cause of LDE. Positive results on the lymphocyte transformation test (LTT) have not been reported in patients with LDE. In the present case, we performed LTT and a patch test, and successfully proved CS as the offending drug in this patient, who had been treated with multiple anti-Tb drugs. These observations suggest that CS should be considered a possible cause of LDE and that LTT can be an option for the diagnosis of LDE.
Lichenoid drug eruption (LDE), also known as drug-induced lichen planus, is an uncommon form of delayed-type drug eruption, but well characterized both clinically and histopathologically.123 LDE has the clinical features of lichen planus, such as widely distributed symmetric skin rashes with or without Wickham striae, typical oral lesions, and similar histopathological findings (including epidermal parakeratosis and lymphocyte infiltration into deeper layers of the dermis), but only LDE shows an elevated eosinophil number.2 The pathogenesis of LDE is type IV hypersensitivity, and CD8+ cytotoxic T cells may secrete TNF-α-triggering keratinocyte apoptosis.4 A diagnosis of LDE is made based on the presence of the suspected causative agent, resolution of symptoms upon discontinuing the causative agent, as well as on clinical and histopathological features.3 LDE accounts for 1% of all cases of drug eruption and is caused by many agents, including gold, quinidine, hydrochlorothiazide, captopril, carbamazepine, and propranolol.25
LDE induced by anti-tuberculosis (anti-Tb) drugs has been reported since Frentz et al.6 first reported ethambutol (EMB)-induced LDE in 1981. EMB, pyrazinamide, rifampicin, isoniazid, and other agents have been reported as causative agents of LDE.1789 However, cycloserine (CS) is a rare cause of LDE,8 and the lymphocyte transformation test (LTT) has not yielded positive results in patients with LDE. Here, we describe a patient who developed CS-induced LDE, which was supported by a patch test and LTT.
A 38-year-old man with pulmonary Tb, who had been treated with anti-Tb medications for 4 months, was referred due to lichenoid eruption and pruritus on the whole body. He was initially treated with isoniazid, rifampin, EMB, and pyrazinamide during the first 2 months and then by second-line anti-Tb medications, including EMB, levofloxacin, and CS, due to reddish itchy papules and hepatitis. One month after commencement of second-line medications, itching became aggravated and lichenoid eruptions developed. On clinical examination, there were widespread hyperkeratotic lesions with scales on the whole body (Fig. 1) and Wickham striae involving the buccal mucosa. There was a non-tender, movable left inguinal lymph node 2 cm in size. The patient had low-grade fever (37℃-38℃) and blood hypereosinophilia (20,640/µL). There were no other abnormalities, such as atypical lymphocytes, except for eosinophilia on blood smear and serum total Immunoglobulin E (IgE) was 1.7 U/mL. Parasite enzyme-linked immunosorbent assay (Toxocara, Paragonimus westermani, Clonorchis sinensis, Sparganum, and Cysticercus) and stool examination were negative. The results of echocardiography, liver enzyme and renal function tests, and urinalysis were all within the normal range. After cessation of anti-Tb medications, the lichenoid skin lesions improved rapidly and blood eosinophilia improved over 3-4 weeks without additional treatment, such as systemic steroids (4,795/µL at 3 weeks and 2,403/µL at 4 weeks; Supplementary Figure). There were no more anti-Tb medications afterward, and the patient was followed up for 1 year without Tb recurrence and flare up of skin lesion.
A patch test and LTT were performed 3 months after stopping medications when the skin had returned to normal. The patch test showed weak positive results for EMB (10%, 50%) and strong positive results for CS (10%, 50%);810 the agents were prepared with white petroleum and showed negative results in 1 healthy control (Fig. 2). LTT, using Cell Counting Kit-8 (CCK-8) as described in the supplemental methods,11 revealed proliferation of peripheral mononuclear cells (PBMCs) by CS in a dose-dependent manner (Fig. 3).
In this case, LDE was prominent 1 month after exposure to CS and presented on the whole body with Wickham striae. There were blood eosinophilia and low-grade fever, which were rapidly improved merely by stopping medications. Only 1 case has been reported for CS-induced LDE confirmed by a patch test, in which the latent period was 5 months and the presence of blood eosinophilia was not obvious.8 Tan et al.12 reported that LDE accounted for 10% of cutaneous adverse drug reactions during treatment with first-line anti-Tb drugs. EMB has been reported as the most common anti-Tb agent responsible for LDE. Previous reports indicated that the latent period between exposure and development of LDE is 3-6 months for EMB,113 1.5 months for pyrazinamide,1314 and a few days for rifampicin.9 Blood eosinophilia has been reported in some cases.91314 In the Korean literature, of 8 cases of LDE, 5 were induced by anti-Tb drugs showed blood eosinophilia and 2 showed Wickham striae.13 LDE is typically resolved simply by cessation of the causative drugs, and thus LDE should be considered when patients receiving anti-Tb treatment present with blood eosinophilia and typical hyperkeratotic skin lesions with scales. In contrast, drug-induced hypersensitivity syndrome shows typically maculopapular skin eruption with blood eosinophilia and a prolonged course requiring long-term systemic steroid treatment.
This is the first report of LTT for LDE that showed results compatible with those of patch tests. LTT has been used to examine the side effects caused by various anti-Tb drugs,15 but this has not been the case for LDE.516 Cutaneous adverse drug reactions, including LDE, are common, developing in 6% of patients treated with anti-Tb drugs,12 and closely related to treatment failure and mortality.17 However, it is not easy to identify the culprit drug as anti-Tb drugs are used in combination for several months. In vitro LTT could identify the culprit drug with minimizing the cessation of medications.15 In this case, LTT demonstrated that the role of CS was obvious in LDE, but it is possible that EMB is one of causative agents for LDE or papular skin lesions during first-line anti-Tb medications, since EMB showed mild positive results in a patch test. LTT was performed using CCK-8 in this case. Although there are no definitive criteria for use of the CCK-8 assay for LTT, unlike for thymidine-uptake assay, the CCK-8 assay has been used to evaluate differences in cell proliferation based on the significance of differences among groups.11
In conclusion, we reported a rare case of CS-induced LDE supported by LTT. This case suggests that CS may be a possible causative agent of LDE and that LTT can be a possible option for the diagnosis of LDE due to CS.
Figures and Tables
Fig. 1
Clinical features of LDE. (A) There was diffuse lichenoid eruption and widespread hyperkeratotic lesions with scales involving the trunk and extremities. (B) Three months after cessation of anti-tuberculosis medications. LDE, lichenoid drug eruption.
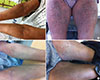
Fig. 2
Patch test with EMB (10%, 50%), CS (10%, 50%), and levofloxacin. Patch test showed weak positive results for EMB (10%, 50%) and strong positive results for CS (10%, 50%). EMB, ethambutol; CS, cycloserine.

Fig. 3
LTT using a CCK-8. (A) PBMCs stimulated with CS, EMB, and sham. Data are from triplicate assays and error bars indicate the standard error. (B) Cell proliferation (%) increased in a dose-dependent manner by CS stimulation. The numbers of cells were 1.70×106, 2.00×106, 2.22×106, and 2.02×106 for sham, cyclosporine 100 µg/mL, cyclosporine 500 µg/mL, and PHA as a positive control, respectively. Data are from triplicate assays and error bars indicate the standard deviation. *P=0.005 (Sham vs CS); **P=0.008 (Sham vs CS). LTT, lymphocyte transformation test; CCK-8, Cell Counting Kit-8; PBMCs, the proliferation of peripheral mononuclear cells; CS, cycloserine; EMB, ethambutol; PHA, phytohemagglutinin.

References
1. Grossman ME, Warren K, Mady A, Satra KH. Lichenoid eruption associated with ethambutol. J Am Acad Dermatol. 1995; 33:675–676.
2. Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. 1993; 29:249–255.
3. Payette MJ, Weston G, Humphrey S, Yu J, Holland KE. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015; 33:631–643.
4. Sugermann PB, Savage NW, Seymour GJ, Walsh LJ. Is there a role for tumor necrosis factor-alpha (TNF-alpha) in oral lichen planus? J Oral Pathol Med. 1996; 25:219–224.
5. Kolm I, Eggmann N, Kamarashev J, Kerl K, French LE, Hofbauer GF. Lichenoid drug eruption following intravenous application of orally formulated diamorphine, a semisynthetic heroin. Case Rep Dermatol. 2013; 5:176–180.
6. Frentz G, Wadskov S, Kassis V. Ethambutol-induced lichenoid eruption. Acta Derm Venereol. 1981; 61:89–91.
7. Thakur BK, Verma S, Mishra J. Lichenoid drug reaction to isoniazid presenting as exfoliative dermatitis in a patient with acquired immunodeficiency syndrome. Int J STD AIDS. 2015; 26:512–515.
8. Shim JH, Kim TY, Kim HO, Kim CW. Cycloserine-induced lichenoid drug eruption. Dermatology. 1995; 191:142–144.
9. Shahul HA, Manu MK, Mohapatra AK, Mathew M. Rifampicin-induced lichenoid eruptions. BMJ Case Rep. 2014; 2014:bcr2013202346.
10. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A;. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001; 45:321–328.
11. Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010; 9:67–78.
12. Tan WC, Ong CK, Kang SC, Razak MA. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007; 62:143–146.
13. Na CH, Seo HD, Chung BS, Shin BS. A case of lichenoid drug eruption with whole body and oral mucosal involvement caused by antituberculosis drugs. Korean J Dermatol. 2008; 46:1145–1148.
14. Choonhakarn C, Janma J. Pyrazinamide-induced lichenoid photodermatitis. J Am Acad Dermatol. 1999; 40:645–646.
15. Suzuki Y, Miwa S, Shirai M, Ohba H, Murakami M, Fujita K, et al. Drug lymphocyte stimulation test in the diagnosis of adverse reactions to antituberculosis drugs. Chest. 2008; 134:1027–1032.
16. Laine J, Happonen RP, Vainio O, Kalimo K. In vitro lymphocyte proliferation test in the diagnosis of oral mucosal hypersensitivity reactions to dental amalgam. J Oral Pathol Med. 1997; 26:362–366.
17. Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996; 9:2026–2030.
18. Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, et al. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci U S A. 2002; 99:3609–3614.
Supplemental Methods
LTT using Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, St. Louis, MO, USA) was performed as follows. Peripheral mononuclear cells (PBMCs) were separated according to their density using Ficoll (Ficoll-Paque PLUS; GE Healthcare, Chalfont, UK). The heparinized blood sample, minus 10 mL kept to obtain auto-plasma as described below, was diluted 3-fold with serum-free RPMI1640 and gently poured into a 50-mL conical tube on top of the Ficoll layer. This solution was centrifuged at 1,120×g at R.T. for 20 minutes. PBMC and the medium layer were transferred to new tubes and centrifuged at 750×g for 10 minutes; the supernatant was discarded to obtain the PBMC pellet. PBMCs were washed with serum-free RPMI 1640 and centrifuged at 750×g for 10 minutes. The supernatant was removed and the cell pellet was resuspended for cell counting and seeding. The remaining 10 mL of blood was centrifuged at 930×g at room temperature for 10 minutes to obtain auto-plasma. The auto-plasma was transferred to a fresh tube and centrifuged at 750×g. Over 3 mL of the upper auto-plasma layer was collected and stored in 1-mL aliquots. PBMCs were seeded into a 96-well plate at 2×105 cells/well (2×106 cells/mL in RPMI 1640) and 10% auto-plasma was added. Cells were stabilized at 37℃ in a 5% CO2 incubator for 2 hours. Ethambutol (EMB), cycloserine (CS), levofloxacin (LF, as a negative control), and phytohemagglutinin (PHA, as a positive control) were added to the wells. EMB, CS, and LF were added at various concentrations (final 0, 10, 100, 500 µg/mL) and PHA was added at 2 µg/mL. Proliferation of PBMCs was measured 3 and 5 days after adding stimulators by CCK-8 according to the manufacturer's protocol.11,18 On the appropriate day, 110 µL of cell culture supernatant was collected from each well to measure the cytokines, and then 10 µL of CCK-8 solution was added. After incubation for 4 hours at 37℃ in a 5% CO2 incubator, the absorbance was measured at 450 nm using a microplate reader.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download