Abstract
Purpose
Asthma is a chronic airway disease characterized by airway remodeling, leading to a progressive decline in lung function. Therapeutic agents that attenuate airway remodeling can complement the limited effects of traditional glucocorticoids. In this study, we investigated the effect of resveratrol on allergic airway inflammation and remodeling in a murine model of chronic bronchial asthma.
Methods
Peribronchial smooth muscle thickening that developed in mice challenged with a 3-month repeated exposure to ovalbumin (OVA) was used to study airway remodeling. Oral resveratrol was administered daily during the OVA challenge. The expression of TGF-β1/Smad signaling proteins and downstream mesenchymal markers in the presence or absence of resveratrol was examined in bronchial epithelial cells.
Results
OVA sensitization and chronic challenge increased airway hyperresponsiveness, inflammation, goblet cell hyperplasia, α-smooth muscle actin (SMA), and collagen deposition. Resveratrol effectively suppressed OVA-induced airway inflammation and remodeling. The expression of TGF-β1/phosphorylated Smad2/3 was increased in the lung tissues of OVA-challenged mice but effectively inhibited by resveratrol. In bronchial epithelial cells, the TGF-β1-induced expression of the mesenchymal markers snail, slug, vimentin, and α-SMA was suppressed by resveratrol treatment.
Conclusions
Resveratrol effectively ameliorated both airway inflammation and airway structural changes in a mouse model of bronchial asthma. These effects were mediated by decreased TGF-β1 expression, in turn suppressing TGF-β1/Smad signaling and the epithelial-mesenchymal transition process. Our results demonstrate the potential benefits of resveratrol for the treatment of airway remodeling associated with bronchial asthma.
Asthma is a chronic airway disease characterized by airway remodeling. The related structural changes consist of subepithelial fibrosis, myofibroblast and myocyte hyperplasia, and an increase in smooth muscle fibers.12 Airway remodeling results in lung function decline and is associated with a lack of responsiveness to treatment. Recently, the traditional concept of airway remodeling, as a consequence of long-standing airway inflammation, has shifted. Instead, airway remodeling is now thought to proceed in parallel with disease development.3456 At the same time, the treatment strategy for asthma is changing because of the limited ability of inhaled corticosteroids to reverse airway remodeling.3789
Epithelial-mesenchymal transition (EMT) describes the differentiation of a polarized epithelium, attached to the basal membrane, into fibroblast-type mesenchymal cells.10 EMT is a physiological process that plays a role in embryogenesis, healing, and repair. However, EMT is also involved in pathologic conditions, such as cancer and abnormal fibrosis seen in idiopathic pulmonary fibrosis. Moreover, studies of the bronchial epithelium have suggested that abnormal EMT is involved in bronchial asthma.21112
Resveratrol (trans-3,4,5-trihydroxystilbene) is a natural polyphenol found in various fruits and vegetables, and in abundance in grapes. In addition to its anti-cancer, anti-inflammatory, and anti-oxidant effects,13 resveratrol has been shown to protect against airway inflammation and remodeling in a mouse model of asthma.1415 These effects of resveratrol have been mainly explained by its inhibition of the cyclooxygenase pathway and nuclear factor kappa B mediated by the inhibition of IκB kinase.13 Moreover, evidence that resveratrol inhibiting the transforming growth factor (TGF)-β1 signaling pathway leads to EMT induction is emerging. For example, resveratrol has been shown to suppress the invasion and metastasis of lung cancer cells and colorectal cancer cells by inhibiting TGF-β1 induced EMT.1617 In renal tubular epithelial cells, resveratrol attenuates renal injury and fibrosis by inhibiting TGF-β1-induced EMT signaling and metalloproteinase 7.18 In addition, the suppressive effects of natural compounds on airway remodeling in an animal model of bronchial asthma have been described. These compounds include kaempferol, triptolide, praeruptorin, and sesamin, which act as inhibitors of TGF-β1/Smad signaling.192021
In this study, we investigated the effects of resveratrol on airway inflammation and remodeling in a murine model of chronic bronchial asthma based on repeated ovalbumin (OVA) challenge and in a bronchial epithelial cell line. Specifically, we assessed whether resveratrol could inhibit TGF-β1/Smad signaling and EMT.
Female 7-week-old BALB/c mice (Dae-Han Experimental Animal Center, Daejon, Korea) were used in this study. The mice were randomly assigned to 1 of the 4 following: (1) control, (2) OVA challenge, (3) OVA challenge plus 10 mg resveratrol/kg administered orally, and (4) OVA challenge plus 50 mg resveratrol/kg administered orally.
The mice were immunized by subcutaneously injecting with 25 µg of OVA (grade V; Sigma-Aldrich, St. Louis, MO, USA) adsorbed to 1 mg of aluminum hydroxide (Aldrich, Milwaukee, WI, USA) in 200 µL of phosphate-buffered saline (PBS). The injections were administered on days 0, 7, 14, and 21. Intranasal challenge with 20 µg OVA/50 µL PBS was administered on days 27, 29, and 31 to mice under isoflurane (Vedco, St. Joseph, MO, USA) anesthesia. The intranasal OVA challenges were then repeated twice a week for 3 months. Age- and sex-matched control mice were treated identically but with PBS alone. All of the mice were euthanized 24 hours after the final OVA challenge, at which time bronchoalveolar lavage (BAL) fluid and lung tissues were obtained. All of the animal research procedures were conducted in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Experiments mandated by the Institutional Animal Care and Use Committee of the School of Medicine, The Catholic University of Korea.
Resveratrol (Sigma, R5010) was prepared as a 100 mM stock solution in ethanol and stored at -20℃. Allergy-sensitized mice were treated by oral gavage with 0.2 mg of saline containing resveratrol (10 or 50 mg/kg) once a day starting on day 35 for 3 months. Control mice were treated with normal saline in the same way.
Airway hyperresponsiveness (AHR) to methacholine (Mch; Sigma-Aldrich) was measured 24 hours after the final OVA inhalation using the flexiVent system (SCIREQ, Montreal, Quebec, Canada) as previously described.22 Briefly, the mice were anesthetized with an intraperitoneal injection of a 1:4 mixture of Rompun and Zoletil. After exposure and cannulation of the trachea, the mice were connected to a computer-controlled small-animal ventilator and ventilated with a tidal volume of 10 mL/kg at a frequency of 150 breaths/min and a positive endexpiratory pressure of 2 cm H2O, to achieve a mean lung volume close to that during spontaneous breathing. Each mouse was administered PBS, followed by increasing concentrations of Mch (6.25, 12.5, 25, 50 mg/mL) in PBS. The peak airway response to the inhaled Mch was recorded.
Immediately after the AHR measurement, BAL was performed by instillation into the lung of 1 mL of sterile PBS through the trachea. The total number of cells in the BAL fluid was counted using a hemacytometer. The BAL fluid was cytospun (7 minutes at 2,000 rpm), placed onto microscope slides, and stained with Diff-Quick (Sysmax, Kobe, Japan). The percentages of macrophages, eosinophils, lymphocytes, and neutrophils in the BAL fluid were determined by counting 400 leukocytes in randomly selected areas of the slide using light microscopy. The supernatants were stored at -70℃.
Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) analysis of TGF-β1 gene expression was carried out using total RNA, isolated from lung homogenates, and TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The primer sequences for the TGF-β1 gene were: forward 5'-GGACTCTCCACCTGCAAGAC-3', reverse 5'-GACTGGCGAGCCTTAGTTTG-3'. Total RNA was extracted from the cells using the Trizol reagent in accordance with the manufacturer's protocol. One microgram of RNA was then reverse-transcribed directly using a PrimeScript™ first-strand cDNA synthesis kit (RR037A, TaKaRa, Tokyo, Japan) according to the manufacturer's protocol. The RT samples were incubated at 37℃ for 15 minutes, inactivated at 85℃ for 5 seconds, and then cooled at 4℃. Thereafter, qRT-PCR was performed using a SYBR® FAST qPCR kit Master Mix (2×) Universal (KR0389, KAPA Biosystems, Boston, MA, USA) with the forward and reverse primers for E-cadherin, snail, slug, vimentin, and GAPDH. PCRs were carried out using a real-time PCR apparatus (CFX96 Touch; Bio-Rad, Hercules, CA, USA). The primer sequences were: snail, forward 5'-TCGGAAGCCTAACTACAGCGA-3', reverse 5'-AGATGAGCATTGGCAGCGAG-3'; slug, forward 5'-AAGCA TTTCAACGCCTCCAAA-3', reverse 5'-CCAGAGGGAGTGAA TCCAGATTA-3'; and GAPDH, forward 5'-TGTGTCCGTCGTGGATCTGA-3', reverse 5'-CCTGCTTCACCACCTTCTTGAT-3'. The samples were incubated at 95℃ for 3 minutes, followed by 40 cycles at 95℃ for 5 seconds, and then at 60℃ for 30 seconds. Differences in expression were determined in a 2 delta-delta Ct relative quantitative analysis. The Ct is the fraction cycle number at which the fluorescence generated by the reporter dye exceeds a set level above baseline. When indicated, the target signal was normalized against the relative quantity of GAPDH and expressed as ΔCt=CtTarget-CtGAPDH. The change in the target signal relative to the total amount of genomic DNA was expressed as ΔΔCt=ΔCttreatment-ΔCtcontrol. Relative changes were then calculated as 2-ΔΔCt.
The levels of interleukin (IL)-4, IL-5, and IL-13 were measured in the supernatant of BAL fluid using an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Dissected lung tissues frozen in liquid nitrogen were disrupted using a Polytron homogenizer (Tissue Tearor™, Biospec Products Inc., Bartlesville, OK, USA) and then centrifuged. The proteins were purified from the supernatant, and their concentrations were assessed using the Bradford method.23 SDS-PAGE was carried out on an 8% acrylamide gel to separate the proteins, which were then transferred to a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Little Chalfont, UK). After a blocking step using 10% skimmed milk (BD Difco, Franklin Lakes, NJ, USA), the membrane was incubated overnight with antibodies against TGF-β1, phosphorylated (p)-Smad2, Smad2, p-Smad3 (1:1,000; Cell Signaling Technology, Danvers, MA, USA), Smad4, Smad7, and β-actin (1:1,000; Santa Cruz, CA, USA), washed 3 times with PBS, and incubated with secondary antibody for 2 hours. For the in vitro analyses, the cultured cells were lysed using radio-immunoprecipitation assay (RIPA) cell lysis buffer containing a mixture of protease inhibitors (GenDEPOT, San Jose, CA, USA) and centrifuged at 13,000 rpm for 30 minutes at 4℃. Equal amounts of extracted protein (40 µg/sample) were separated by SDS-PAGE using a 10% polyacrylamide gel in Tris-glycine-SDS buffer and transferred to 0.45-µm PVDF membranes. The membranes were blocked with 5% skim milk prepared in Tris-buffered saline containing 0.05% Tween 20 and incubated overnight with antibodies against snail and slug (1:1,000; Cell Signaling Technology) and α-smooth muscle actin (SMA) (1 µg/mL; Abcam, Cambridge, UK) at 4℃. The membranes were then washed and incubated with a horseradish peroxidase-conjugated secondary antibody. Bands were detected using the western blotting luminol reagent (ELIPIS Biotech., Inc., Daejeon, Korea).
Following the collection of BAL fluid, the mouse lungs were inflated, fixed in 4% paraformaldehyde for 24 hours, and then embedded in paraffin. Sections (4-µm thick) cut using a microtome were stained with hematoxylin and eosin (H&E) using standard histological techniques. The paraffin-embedded tissues were also sectioned (5- to 6-µm thick) and stained with periodic acid-Schiff (PAS) to identify goblet cells in the epithelium. Goblet cell hyperplasia was quantified using the method described by Padrid et al.24 The pathological changes were evaluated according to a modified five-point scoring system (grades 0-4) based on the percentage of goblet cells in the epithelium: grade 0 (no goblet cells); grade 1 (<25%); grade 2 (25%-50%); grade 3 (51%-75%); and grade 4 (>75%). The mean goblet cell hyperplasia score was then calculated for each mouse.
As previously described, α-SMA was immunohistochemically detected.25 The area in each paraffin-embedded lung immunostained by α-SMA was outlined and quantified using a light microscope attached to an image analysis system (BX50; Olympus, Tokyo, Japan). The results were expressed as the immunostained area of the bronchiolar basement membrane (internal diameter 150-200 µm). At least 10 bronchioles were counted in each slide.
The hydroxyproline assay was carried out using 60 mg of homogenized lung tissue from each mouse. The samples were incubated in 250 µL of 12 N HCl for 16 hours at 110℃. After centrifugation, 25 µL of supernatant was incubated for 20 minutes in 25 µL of citrate/acetate buffer (5% citric acid, 7.2% sodium acetate, 3.4% sodium hydroxide, and 1.2% glacial acetic acid) and 500 µL of chloramine T solution (1.41 g of chloramine T, 26 mL of n-propanol, 20.7 mL of distilled water, and 53.3 mL of citrate/acetate buffer), after which 500 µL of Ehrlich's solution (4.5 g of p-dimerthylaminobenzaldehyde, 18.6 mL of n-propanol, and 7.8 mL of 70% perchloric acid) was added. These samples were incubated for 15 minutes at 65℃, cooled, and then analyzed in a spectrophotometer at 550 nm. Hydroxyproline concentrations were calculated from a standard curve of hydroxyproline.
The human bronchial epithelial cell line BEAS-2B was obtained from the American Type Culture Collection (CRL-9609; ATCC, Rockville, MD, USA). The cells were cultured in DMEM/F-12 (1:1) medium (WelGENE Inc., Daegu, Korea) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (10,000 units/mL and 10 mg/mL, respectively) at 37℃ under 5% CO2 in air.
Resveratrol was prepared as described above and then diluted in cell culture medium to obtain working concentrations. The maximum final concentration of ethanol was less than 0.025% for each treatment. Recombinant human TGF-β1 (00-21-10; Peprotech, Rocky Hill, NJ, USA) was prepared as a stock solution at a concentration of 0.1 mg/mL in 10 mM citric acid and stored at -20℃. Cells that had reached 70%-80% confluence were maintained in serum-free DMEM/F-12 for 24 hours and then incubated for 48 hours with 5 µM TGF-β1 in the presence or absence of different concentrations of resveratrol.
The results from each group were compared by ANOVA and the nonparametric Kruskal-Wallis test, followed by post hoc testing with Dunn's multiple comparison of means. All statistical analyses were performed using Graph-Pad Prism for Windows software (ver. 5.00; GraphPad Software, San Diego, CA, USA). A P value of <0.05 was considered to indicate statistical significance. Results are expressed as means±SEM.
Mice in the OVA group showed increased AHR after Mch challenge compared to mice in the control group. In the presence of resveratrol, however, the increased AHR was suppressed (Fig. 1). After a 3-month OVA challenge, the number of inflammatory cells in BAL fluid was higher than in the control (Fig. 2) but reduced by resveratrol treatment. Total cells and eosinophil counts in BAL fluid were significantly lower in the resveratrol-treated group than in the OVA group (P<0.05), as were the levels of IL-4, -5, and -13 (P<0.05) (Fig. 3).
Histopathologic staining using H&E showed that compared to the OVA group, resveratrol effectively suppressed the infiltration of peribronchial inflammatory cells (Fig. 4A). On the PAS-stained sections, the number of goblet cells were decreased with resveratrol treatment (Fig. 4B), and the pathological-change scores were significantly lower (Fig. 4C). Immunostaining of peribronchial α-SMA (Fig. 5) showed that a repetitive 3-month challenge with OVA resulted in an immunostained area of α-SMA that was significantly larger than in the control, an effect that was reduced by resveratrol (P<0.05). A hydroxyproline assay of total lung collagen showed that resveratrol (50 mg/kg) suppressed OVA-challenge-induced hydroxyproline levels (Fig. 6).
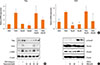
To evaluate the effect of resveratrol in TGF-β1 signaling, the expression of TGF-β1 in BAL fluid and lung tissue samples was evaluated. Fig. 7 shows the level of TGF-β1 in BAL fluid and lung tissues. In BAL fluid, the expression of TGF-β1 was decreased significantly with resveratrol treatment (50 mg/kg) (Fig. 7A); in lung tissues, TGF-β1 mRNA and proteins expression were also suppressed by resveratrol (Fig. 7B and C).
We hypothesize that the mechanism by which resveratrol decreases airway inflammation involved a decrease in TGF-β1 and thus also in its downstream signaling proteins. We therefore examined one of the TGF-β1 signaling pathways associated with the pathophysiology of bronchial asthma, EMT. In the lung tissues of OVA-challenged mice, the expression of TGF-β1, p-Smad2, and p-Smad3 were higher in the absence of reservation and lower in the presence. The suppressive effects of 50 mg resveratrol/kg were significant (P<0.01) (Fig. 7C and D).
To confirm the effects of resveratrol on EMT, we carried out an in vitro study using a bronchial epithelial cell line and evaluated the expression of mesenchymal EMT protein markers by qRT-PCR and Western blotting. As seen in Fig. 8A, the mRNA levels of the mesenchymal markers slug and snail in bronchial epithelial cells were increased in response to TGF-β1 treatment but decreased in the presence of resveratrol. Western blot analysis showed similar changes in the levels of these proteins as well as α-SMA (Fig. 8B). In addition, the protein expression of p-Smad2 and Smad4 was increased with TGF-β1 stimulation and decreased with resveratrol treatment (Fig. 8C).
The results of this study demonstrated the beneficial effects of resveratrol in reducing the bronchial inflammation in OVA-challenged mice in a model of chronic bronchial asthma. The daily oral administration of resveratrol during OVA challenge effectively suppressed AHR, airway inflammation, and the remodeling process. These effects were mediated by inhibiting TGF-β1 signaling and included the suppressed expression of mesenchymal markers and a reduction in the phosphorylation of Smad2/3.
In our search for therapeutic agents targeting airway remodeling, we referred to our published reports demonstrating the suppressive effects of tyrosine kinase inhibitors, such as imatinib and nilotinib, in the OVA-challenged mouse model of chronic bronchial asthma.2526 Resveratrol was investigated as an extension of previous reports of a significant inhibition of goblet cell hyperplasia, smooth muscle proliferation, and total lung collagen levels. In this work, we additionally showed that AHR and airway inflammation, both of which contribute to the remodeling process, are effectively ameliorated by resveratrol treatment. Airway remodeling consists of structural changes in the airway wall, including epithelial injury, subepithelial thickening, airway smooth muscle hyperplasia, goblet cell hyperplasia, and hypertrophy.3 Although these processes result in a progressive and irreversible loss of lung function, current asthma therapies, such as corticosteroids, are ineffective in suppressing airway remodeling.27 The results of our study suggest the use of resveratrol as an additional therapeutic agent that targets not only allergic AHR and inflammation, but also the accompanying, irreversible structural changes in the airways.
Previous reports have described the beneficial effects of resveratrol in different animal models of bronchial asthma. Lee et al.15 showed that resveratrol effectively suppressed AHR, eosinophilia, and mucus hypersecretion in a mouse model of acute asthma. In that study, the efficacy of resveratrol was similar to that of glucocorticoid. Those authors also examined the effect of resveratrol produced in BAL fluid, both in a histopathology study and by measuring cytokine levels; their results were consistent with ours. In a murine model of chronic asthma induced by OVA challenge, Royce et al.14 evaluated the effects of resveratrol with respect to subepithelial collagen deposition, goblet cell hyperplasia, and TGF-β1 immunohistochemistry staining in lung specimens. However, 6-week OVA challenge was insufficient to allow assessment of peribronchial smooth muscle cell proliferation in the lung tissues of control, OVA-challenged, and OVA-challenged, resveratrol-treated mice. This is an important advantage of the asthma model used in our study compared to other animal models of chronic asthma. Other animal models could not demonstrate the changes airway smooth muscle area and total lung collagen level quantitatively. Specifically, following a 12-week OVA challenge, the area of α-SMA coverage was significantly greater than in the control group. This difference was minimized by resveratrol treatment, which effectively suppressed peribronchial smooth muscle proliferation (P<0.05). Our 12-week OVA challenge model is superior to previous reports to explain inhibitory effects of resveratrol on airway remodeling process, more clinically relevant. Moreover, as far as we know, the role of resveratrol in TGF-β1/Smad signaling in bronchial asthma animal model has not been published yet.
TGF-β1 signaling plays a pivotal role in the airway remodeling that characterizes chronic asthma and has been implicated in most of the cellular processes involved, including subepithelial fibrosis, airway smooth muscle remodeling, and epithelial and microvascular changes. The mechanism by which TGF-β1 induces structural changes in bronchial asthma includes the TGF-β1/Smad pathway and TGF-β1-induced EMT. Under stimulation by TGF-β1, Smad proteins transport signals from the cell membrane to the nucleus, targeting genes that regulate cellular proliferation, transformation, and synthesis. It has been known that Smad2 and Smad3 are receptor regulated Smads (R-Smad) which are phosphorylated by the activation of TGF-β signaling. Smad4 is a common pathway Smad, which cooperates to phosphorylate Smad2/3. Smad6 and Smad7 are inhibitory Smads (I-Smad) which down-regulate TGF-β signaling.28 Smad2 and Smad3 must be phosphorylated to be active, whereas Smad7 inhibits their phosphorylation.2930 In vivo evidence of a role for the TGF-β1/Smad pathway in bronchial asthma includes increased levels of TGF-β1 and p-Smad2/3, and a decrease in Smad7 in the lung tissues of OVA-sensitized mice.1920 In our study, we examined the balance of Smad signaling evoked by OVA with or without resveratrol. We observed the expression of Smad2, 3, and 4 mRNA and proteins in BEAS-2B cells as well as mouse lung tissues. Increased levels of phospho-Smad2 by OVA and Smad4 by TGF-β were decreased by resveratrol treatment. However, expression of inhibitory Smad7 protein was not changed with resveratrol treatment compared to ovalbumin control (data not shown). Based on these data, we found that resveratrol could effect on OVA-induced mice model through the TGF-β/Smad activation pathway. The contribution of EMT to airway fibrosis and remodeling in epithelial cells has been demonstrated by in vitro and in vivo studies. The EMT process can be followed by measuring the expression of epithelial or mesenchymal markers. In an in vitro study carried out Yang et al.,31 TGF-β1-stimulated EMT was monitored in human bronchial epithelial cells. TGF-β1 treatment decreased the expression of the epithelial marker E-cadherin and increased that of the mesenchymal markers snail, vimentin, and α-SMA. The artificial down-regulation of snail attenuated the TGF-β1-induced EMT phenotype. Gong et al.32 reported the inhibitory effect of kaempferol on EMT in OVA-sensitized mice. They showed that in the mouse trachea, expression of TGF-β1 and the mesenchymal marker α-SMA were increased in OVA-sensitized mice but decreased in mice treated with kaempferol. Our results correspond well with studies reporting elevated TGF-β1 expression in OVA-challenged mice and TGF-β1-mediated stimulation of the mesenchymal transition in bronchial epithelial cells. Additionally, we found that both TGF-β1 expression and Smad2/3 phosphorylation were decreased significantly in the lung tissues of OVA-challenged mice administered resveratrol intraorally. Moreover, the TGF-β1-induced stimulation of the mesenchymal markers slug, snail, and α-SMA as well as Smad2/4 proteins in bronchial epithelial cells were effectively suppressed by resveratrol treatment.
Since the EMT is a dynamic process, Western blots of epithelial or mesenchymal markers provide only a "snapshot." The reverse phenomenon, i.e., mesenchymal-epithelial transition, can occur simultaneously and the 2 processes may influence each other.11 Thus, experimental methods able to track both are needed.
Taken together, in this study the potential therapeutic effect of resveratrol in the treatment of bronchial asthma was investigated. The results showed that the oral administration of resveratrol effectively suppressed allergic airway inflammation and remodeling in lung tissues. These effects of resveratrol were mediated by suppressing of TGF-β1 expression, downstream Smad activation in lung tissues, and mesenchymal transition in bronchial epithelial cells. Therefore, by targeting the airway remodeling process resveratrol may be an effective therapeutic agent against bronchial asthma.
Figures and Tables
Fig. 1
Effect of resveratrol on airway hyperresponsiveness (AHR) to methacholine (Mch). AHR was measured using the flexiVent system 24 hours after the final ovalbumin (OVA) challenge. The concentration of Mch was increased from 6.25 to 50 mg/mL. OVA, OVA-sensitized/challenged mice; RES (10 mg/kg), 10 mg resveratrol/kg+OVA; RES (50 mg/kg), 50 mg resveratrol/kg administered to sensitized/challenged mice. The values are means±SEM (n=5/group). *P<0.05 vs the OVA group.

Fig. 2
Effect of resveratrol on total and differential cell counts in bronchoalveolar lavage (BAL) fluid. BAL was performed immediately after the measurement of AHR. OVA, OVA-sensitized/challenged mice; RES (10 mg/kg), 10 mg resveratrol/kg+OVA; RES (50 mg/kg), 50 mg resveratrol/kg administered to sensitized/challenged mice. The values are means±SEM (n=8-12/group). *P<0.05 vs the OVA group.
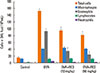
Fig. 3
Effects of resveratrol on cytokine levels in BAL fluid. Interleukin (IL)-4, IL-5, and IL-13 levels in the supernatant of BAL fluid of OVA-sensitized/challenged mice were measured using an ELISA. RES (10 mg/kg), 10 mg resveratrol/kg+OVA; RES (50 mg/kg), 50 mg resveratrol/kg administered to sensitized/challenged mice. The values are means±SEM (n=8-12/group). *P<0.05 vs the OVA group.

Fig. 4
Effects of resveratrol on peribronchial inflammation in lung tissue. (A) The fixed tissues were embedded in paraffin, sectioned (4-µm thick), and then stained with H&E (×200). (B) The effects of resveratrol on goblet cell hyperplasia in lung tissue. Paraffin-embedded tissues were sectioned (5- to 6-µm thick) and then stained with periodic acid-Schiff (PAS). (C) Goblet cell hyperplasia was quantified based on the percentage of goblet cells in the epithelium, scored as follows: grade 0 (no goblet cells); grade 1 (<25%); grade 2 (25%-50%); grade 3 (51%-75%); grade 4 (>75%). The mean goblet cell hyperplasia score was calculated for each mouse (n=8-12/group). OVA, OVA-sensitized/challenged mice; RES (10 mg/kg), 10 mg resveratrol/kg+OVA; RES (50 mg/kg), 50 mg resveratrol/kg administered to sensitized/challenged mice. *P<0.05 vs the OVA group.
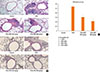
Fig. 5
Effects of resveratrol on area of peribronchial smooth muscle. (A) Peribronchial α-smooth muscle actin was immunostained in paraffin-embedded lung sections (red). (B) The immunostained area was quantified using an image analysis system. The results are expressed as the immunostained area per unit basement membrane length (µm) in the bronchioles of each mouse (n=8-12/group). OVA, OVA-sensitized/challenged mice; RES (10 mg/kg), 10 mg resveratrol/kg+OVA; RES (50 mg/kg), 50 mg resveratrol/kg administered to sensitized/challenged mice. *P<0.05 vs the OVA group.

Fig. 6
Effect of resveratrol on total collagen levels in lung tissue (60 mg/mouse) determined by hydroxyproline assay. OVA, OVA-sensitized/challenged mice; RES (10 mg/kg), 10 mg resveratrol/kg+OVA; RES (50 mg/kg), 50 mg resveratrol/kg administered to sensitized/challenged mice. The values are means±SEM (n=8-12/group). *P<0.05 vs the OVA group.
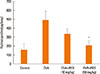
Fig. 7
Effect of resveratrol on the expression of TGF-β1 and phosphorylated (p)-Smad in BAL fluid and lung tissues. (A) TGF-β1 expression was measured in BAL fluid by ELISA. *P<0.05 vs the OVA group. (B) Quantitative real-time reverse-transcription polymerase change reaction (qRT-PCR) analysis of TGF-β1 gene in homogenized lung tissues. *P<0.05 vs the OVA group. (C and D) Expression of TGF-β1, p-Smad2, and p-Smad3 proteins in lung tissues as determined by western blotting. OVA, OVA-sensitized/challenged mice; RES (10 mg/kg), 10 mg resveratrol/kg+OVA; RES (50 mg/kg), 50 mg resveratrol/kg administered to sensitized/challenged mice. The values are means±SEM (n=8-12/group). *P<0.05 and †P<0.01 vs the control group, ‡P<0.05 and §P<0.01 vs the OVA group, ∥P<0.01 vs the OVA+RES10 group.
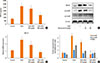
Fig. 8
Effects of resveratrol on the expression of epithelial-mesenchymal transition (EMT) protein markers and Smad proteins in BEAS-2B cells treated for 48 hours with 5 µM TGF-β1 with or without 10 or 20 µM of resveratrol. (A) The expression of snail and slug mRNA was measured by quantitative reverse transcription (qRT)-PCR. (B) Mesenchymal markers (slug, snail and α-smooth muscle actin) were examined by western blot analysis. (C) The expression of Smad2, p-Smad2 and Smad4 proteins were determined by western blotting. CON, control; TGFβ, TGF-β1 (5 µM) treated only; Res10, treated with 10 µM resveratrol; Res20, 20 µM resveratrol; TGFβ+Res10, TGF-β1 and resveratrol 10 µM; TGFβ+Res20, TGF-β1 and resveratrol 20 µM. *P<0.05 and †P<0.01 vs the TGF-β1-treated cells. ‡P<0.05 vs TGF-β+resveratrol (10 µM)-treated cells.
References
1. Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003; 111:291–297.
2. Pain M, Bermudez O, Lacoste P, Royer PJ, Botturi K, Tissot A, et al. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev. 2014; 23:118–130.
3. Durrani SR, Viswanathan RK, Busse WW. What effect does asthma treatment have on airway remodeling? Current perspectives. J Allergy Clin Immunol. 2011; 128:439–448.
4. Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol. 2013; 31:3–10.
5. Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014; 6:376–388.
6. Sposato B, Scalese M, Migliorini MG, Di Tomassi M, Scala R. Small airway impairment and bronchial hyperresponsiveness in asthma onset. Allergy Asthma Immunol Res. 2014; 6:242–251.
7. Makino S, Sagara H. Evolution of asthma concept and effect of current asthma management guidelines. Allergy Asthma Immunol Res. 2010; 2:172–176.
8. Chang WS, Kim EJ, Lim YM, Yoon D, Son JY, Park JW, et al. Age-related changes in immunological factors and their relevance in allergic disease development during childhood. Allergy Asthma Immunol Res. 2016; 8:338–345.
9. Chen Y, Wong GW, Li J. Environmental exposure and genetic predisposition as risk factors for asthma in China. Allergy Asthma Immunol Res. 2016; 8:92–100.
10. Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005; 233:706–720.
11. Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014; 69:760–765.
12. Hackett TL. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol. 2012; 12:53–59.
13. Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal. 2010; 13:1535–1548.
14. Royce SG, Dang W, Yuan G, Tran J, El Osta A, Karagiannis TC, et al. Resveratrol has protective effects against airway remodeling and airway hyperreactivity in a murine model of allergic airways disease. Pathobiol Aging Age Relat Dis. 2011; 1:7134.
15. Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009; 9:418–424.
16. Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, et al. Resveratrol inhibits TGF-beta1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology. 2013; 303:139–146.
17. Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, et al. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015; 15:97.
18. Xiao Z, Chen C, Meng T, Zhang W, Zhou Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-beta pathway on matrix metalloproteinase 7. Exp Biol Med (Maywood). 2016; 241:140–146.
19. Chen M, Lv Z, Jiang S. The effects of triptolide on airway remodelling and transforming growth factor-beta(1)/Smad signalling pathway in ovalbumin-sensitized mice. Immunology. 2011; 132:376–384.
20. Xiong YY, Wang JS, Wu FH, Li J, Kong LY. The effects of (+/-)-Praeruptorin A on airway inflammation, remodeling and transforming growth factor-beta1/Smad signaling pathway in a murine model of allergic asthma. Int Immunopharmacol. 2012; 14:392–400.
21. Lin CH, Shen ML, Kao ST, Wu DC. The effect of sesamin on airway fibrosis in vitro and in vivo. Int Immunopharmacol. 2014; 22:141–150.
22. Tarkowski M, Vanoirbeek JA, Vanhooren HM, De Vooght V, Mercier CM, Ceuppens J, et al. Immunological determinants of ventilatory changes induced in mice by dermal sensitization and respiratory challenge with toluene diisocyanate. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L207–L214.
23. Kruger NJ. The Bradford method for protein quantitation. Methods Mol Biol. 1994; 32:9–15.
24. Padrid P, Snook S, Finucane T, Shiue P, Cozzi P, Solway J, et al. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am J Respir Crit Care Med. 1995; 151:184–193.
25. Rhee CK, Kim JW, Park CK, Kim JS, Kang JY, Kim SJ, et al. Effect of imatinib on airway smooth muscle thickening in a murine model of chronic asthma. Int Arch Allergy Immunol. 2011; 155:243–251.
26. Rhee CK, Kang JY, Park CK, Lee SY, Kwon SS, Kim YK, et al. Effect of nilotinib on airway remodeling in a murine model of chronic asthma. Exp Lung Res. 2014; 40:199–210.
27. Royce SG, Tang ML. The effects of current therapies on airway remodeling in asthma and new possibilities for treatment and prevention. Curr Mol Pharmacol. 2009; 2:169–181.
28. Groneberg DA, Witt H, Adcock IM, Hansen G, Springer J. Smads as intracellular mediators of airway inflammation. Exp Lung Res. 2004; 30:223–250.
29. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003; 425:577–584.
30. Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ, Ran N. Inhibition airway remodeling and transforming growth factor-beta1/Smad signaling pathway by astragalus extract in asthmatic mice. Int J Mol Med. 2012; 29:564–568.
31. Yang ZC, Yi MJ, Ran N, Wang C, Fu P, Feng XY, et al. Transforming growth factorbeta1 induces bronchial epithelial cells to mesenchymal transition by activating the Snail pathway and promotes airway remodeling in asthma. Mol Med Rep. 2013; 8:1663–1668.
32. Gong JH, Cho IH, Shin D, Han SY, Park SH, Kang YH. Inhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab Invest. 2014; 94:297–308.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download