Abstract
Purpose
Limited data is available on the prevalence and risk factors of acute and chronic urticaria in children. Our purpose was to determine the prevalence and identify the risk factors of acute and chronic urticaria in Korean children.
Methods
This population-based study examined 4,076 children (age 4 to 13 years) who were enrolled in the 2015 prospective Seongnam Atopy Project (SAP 2015) in Korea. The parents completed an urticaria questionnaire that included questions regarding the duration, severity, and triggering factors of urticaria. Blood sampling (n=464) was performed to measure vitamin D, total eosinophil count (TEC), and total IgE levels, and skin prick tests (n=503) were done.
Results
The prevalences of the life-time, acute, and chronic urticaria were 22.5%, 13.9%, and 1.8% (chronic continuous urticaria, 0.7%; and chronic recurrent urticaria, 1.1%), respectively. Acute urticaria was significantly associated with allergic diseases and parental history of allergy (P<0.001), but chronic urticaria was not associated with these clinical factors. There was no significant difference in the 25-hydroxyvitamin D level between subjects with chronic urticaria and controls (P=0.124). Chronic continuous urticaria was associated with living in a new residence (aOR=2.38, 95% CI=1.02-5.54, P=0.044) and belonging to a family with a high income (aOR=4.24, 95% CI=1.24-14.56, P=0.022).
Urticaria is one of the most common skin diseases, and patients may present with a wide spectrum of symptoms, including sudden development of pruritic wheals or angioedema.1 Chronic urticaria has a significant impact on quality-of-life due to the constant sensation of itching, regular recurrence, and unknown etiology.2 Urticaria is classified as acute or chronic form based on the duration of illness. Chronic urticaria is diagnosed when disease has been continuously or intermittently present for at least 6 weeks.3 The chronic and acute forms of urticaria differ in etiology, pathophysiology, and underlying mechanism.3
An estimated 15% to 23% of adults have experienced at least 1 episode of acute urticaria at some time in their lives,45 and the prevalence of chronic urticaria in adults is estimated at 0.5% to 5%.236 In children, previous studies reported the prevalence of acute urticaria to be 1% to 14.5%,178 but there is little data on the prevalence of chronic urticaria in children.57 Chronic urticaria is considered less prevalent in children than in adults,9 and most studies estimating its prevalence are based upon extrapolation from adult data,7 hospital-based results,810 or studies of the general adult population.2
Previous studies have reported that chronic idiopathic urticaria is associated with infection,11 pseudo-allergy to a food,12 autoimmunity,1314 and low serum level of vitamin D.15161718 However, there is little information on the relationship of chronic urticaria with type of residence, overcrowding, socio-economic status, air pollutants, exposure to secondary smoke, and other life style factors that have established importance in other allergic diseases.121920
The aims of this study were to estimate the prevalence of acute and chronic urticaria in children and to identify their associations with the demographic and epidemiological characteristics of individuals, including family history, comorbidities, disease severity, and environmental factors by using questionnaires.
This cross-sectional study examined children from the general pediatric population who were 4 to 13 year-old and attended 3 kindergartens and 6 elementary schools as part of the Seongnam Atopy Project in Korea from June to July of 2015 (SAP2015). We distributed questionnaires to the parents of 5,196 children and received 4,434 completed questionnaires in return (response rate: 85.3%). A total of 358 subjects completed the questionnaire incorrectly (e.g. missing values or absence of written consent) and were excluded. Therefore, 4,076 (78.5%) children were finally included in this study. This study was approved by the Institutional Review Board of the CHA Bundang Medical Center. Written consent was obtained from all parents or guardians following a detailed explanation of the study.
Based on previous studies,121121 “lifetime urticaria” was defined by an affirmative answer to the question: “Has your child had a problem with burning, itching or a rash resembling a mosquito bite or swelling on the skin during his/her lifetime?” Among those with lifetime urticaria, “current urticaria” was defined by an affirmative answer to the question: “In the past 12 months, has your child had a problem with burning, itching, or a rash resembling a mosquito bite or swelling on the skin?” Current urticaria was defined as acute or chronic urticaria according to its duration,322 “Chronic continuous urticaria” was defined by the presence of daily symptoms or outbreaks occurring 3 or more times a week and lasting more than 6 weeks.2 “Chronic recurrent urticaria” was defined by the presence of repeated development of symptoms with intervals of at least a week between each relapse and lasting more than 6 weeks.7 We defined “ever diagnosed with urticaria” by an affirmative answer to the question: “Has your child ever been diagnosed with urticaria at least once by a doctor”. We defined “current treatment” based on an affirmative answer to the question: “Has your child undergone treatment for urticaria during the past year?” Control subjects were children who did not experience urticaria during their lifetimes.
We used the modified urticaria activity score (UAS)23 to assess disease severity. In particular, the severity of urticaria was assessed by asking: “How many wheals were there when your child's urticaria was most severe?” Wheals were classified as none, mild (20 or fewer wheals per 24 hours), moderate (20 to 50 wheals per 24 hours), or intense (50 or more wheals per 24 hours or large confluent areas of wheal). Pruritus was classified as none, mild (present but not annoying or troublesome), moderate (troublesome but not interfering with normal daily activities or sleep), or intense (very troublesome and interfering with normal daily activities or sleep).
We asked about the presence of factors that might induce urticaria, such as exposure to specific foods, drugs, cold exposure (cold water or air), hot air (after a shower or exercise), common cold, changes in the environment, and stress.11
We performed blood sampling to measure white blood cell count, vitamin D, total eosinophil count (TEC), and total IgE levels (Phadia AB, Uppsala, Sweden). We also used skin prick tests (SPTs) in 503 children to assess allergic sensitization to 22 common allergens using extract and control solutions from Lofarma (Milan, Italy): Dermatophagoides pteronyssinus (Der p.), Dermatophagoides farina (Der f.), birch, oak, elm, hop, egg, milk, peanut, wheat, walnut, cod, pork, mussel, shrimp, apple, peach, kiwi, orange, tomato, strawberry, and celery. A subject was considered atopic if there was 1 or more positive reactions (wheal diameter > 3 mm) to any allergen in the SPT.
A modified Korean version of International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire was used to determine the prevalence of sign and/or symptoms and the diagnosis of allergic diseases. This questionnaire consists of 3 main sections: (1) general characteristics, including sex, birth year, height, and weight; (2) history of sign and/or symptoms related to asthma, allergic rhinitis (AR), atopic dermatitis (AD), and food allergy (FA), and family history, including parental asthma, allergic rhinitis, and atopic dermatitis; and (3) environmental factors. The environmental factors were: number of family members living together; presence, number, and birth order of other siblings; total days of activity per week out of physical education class (0, 1-2, 3-6, and 7 days/week), type of delivery (vaginal delivery vs C-section), birth weight, breast feeding, weaning period, attendance at a daycare center (among attendees: age at initial attendance and duration of attendance), exposure to secondary smoke, type of residence (detached house vs apartment), age of the residence (<6 vs ≥6 years), floor number in the residence, presence of mold on the walls or ceiling of the residence, type of ventilation, pet ownership, furniture less than 1 year old, change of residence since birth, remodeling of the residence (replacing wallpaper, flooring or ceiling, etc), distance of the residence to the main road (<50, 50-100, 100-200, >200 m), parental education, and parental income (<$3,360 vs ≥$3,360/month).
Statistical analysis was performed using SPSS version 22.0 (IBM Co, Armonk, NY, USA). The relationships of different parameters with urticaria were determined using Student's t test for continuous variables and Pearson's χ2 test for dichotomous variables. A linear-by-linear (Mantel-Haenszel test) association trend test was used to test the presence of a linear trend of the proportion of urticaria and sex according to age in current urticaria. The agreement between current urticaria and treatment history in the past 12 months, lifetime urticaria, and diagnosis by a physician were analyzed by calculation of κ coefficients. Multivariate logistic regression analysis, after adjustment for age, sex, BMI z-score, and personal history of atopic dermatitis, was used to identify independent risk factors of acute and chronic urticaria (continuous and recurrent types). For all analyses, a P value of <0.05 was considered significant.
Fig. 1 shows the classification of the study subjects based on our criteria for the diagnosis of different types of urticaria. A total of 917 subjects (22.5%, mean age: 9.4±1.7 years) had a wheal or angioedema at some time during their lifetimes, and 624 of them (15.3%) had current urticaria. We further classified subjects with current urticaria as having acute urticaria (n=567, 13.9%), chronic continuous urticaria (n=27, 0.7%), or chronic recurrent urticaria (n=30, 1.1%).
Table 1 shows the characteristics of the 4,076 study subjects. These results indicate that subjects who ever had urticaria were more likely to have asthma, AR, or AD, and that their parents were also more like to have asthma, AR, or AD. The cases and controls had no significant differences in sex, age, height, or BMI z-score.
The percentage of subjects with current urticaria among the total subjects decreased significantly with age (P=0.009). However, within each age group, the sex ratio of subjects with current urticaria (P=0.070) did not differ from that of subjects with chronic urticaria (P=0.735). The κ for agreement between lifetime urticaria and ever diagnosis was 0.389 (P<0.001), and the κ for agreement between current urticaria and current treatment was 0.315 (P<0.001).
Table 2 shows the characteristics of the 624 subjects with current urticaria, which included 567 subjects with acute urticaria and 57 with chronic urticaria. These 2 groups had similar demographics, hematologic profiles, allergic profiles, and parental histories. Interestingly, there was a parental history of AR in 64.5% of those with acute urticaria, 40.7% of those with chronic continuous urticaria, and 69% of those with chronic recurrent urticaria (P=0.036, data not shown). The 25-hydroxyvitamin D levels of subjects with acute urticaria, those with chronic urticaria, and controls were 20.5±5.1, 22.9±4.9, and 20.0±5.1 ng/mL, respectively; there was no significant between-group difference (P=0.183). The percentages of positive skin prick tests in subjects with acute urticaria, those with chronic urticaria, and controls were 58.7%, 54.6%, and 48.3%, respectively; there was no significant between-group difference. The maximum UAS for wheals was significantly higher in subjects with chronic urticaria than in those with acute urticaria (P=0.028) (Fig. 2A), but this relationship was not observed for pruritus (P=0.204) (Fig. 2B). There was no significant difference in the maximum UAS between subjects with chronic recurrent urticaria and those with chronic continuous urticaria (data not shown).
Food was the most frequent cause of urticaria (n=236, 37.8%), followed by changing environment (n=172, 27.6%). There was a significant difference in cold exposure as a cause of urticaria between those with acute and chronic disease (6.7% vs 14%, P=0.043) (Fig. 3). However, there was no significant difference in environmental change including sunlight exposure, exposure to allergen, contact, irritants, pressure, warm exposure, stress, infection, or drug.
We calculated the adjusted odds ratios (aORs) for acute and chronic urticaria relative to controls after adjustment for age, sex, BMI z score, and atopic dermatitis (Table 3). The results showed that parental history of asthma (aOR=1.71, 95% CI=1.15-2.54, P=0.007), parental history of AR (aOR=1.71, 95% CI=1.38-2.10, P<0.001), parental history of AD (aOR=2.07, 95% CI=1.56-2.76, P<0.001), remodeling of the house (aOR=1.32, 95% CI=1.07-1.65, P<0.011), and moving into house (aOR=1.47, 95% CI=1.17-1.84, P<0.001) increased the risk for acute urticaria. Living in a new house was the only factor that increased the risk of chronic urticaria (aOR=2.12, 95% CI=1.17-3.84, P=0.013).
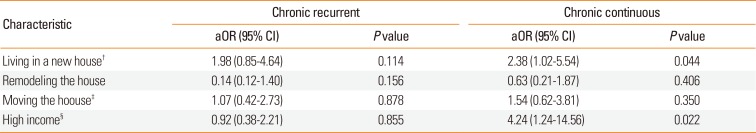
We also calculated the aORs for chronic recurrent urticaria and chronic continuous urticaria relative to controls after adjustment for the same confounders (Table 4). The results showed that living in new house (aOR=2.38, 95% CI=1.02-5.54, P=0.044) and high income (aOR=4.24, 95% CI=1.24-14.56, P=0.022) significantly increased the risk of chronic continuous urticaria (Table 4). No risk factors were significantly associated with increased risk of chronic recurrent urticaria.
We found that acute urticaria in the study population was as prevalent as other allergic diseases, such as asthma and AR. The prevalence of chronic urticaria in children was similar to that previously reported for adults. To the best of our knowledge, this is the first population-based epidemiological study to estimate the prevalence of chronic urticaria in children. There was no difference in the demographic, laboratory parameters (including vitamin D), and allergic profiles of subjects who had acute or chronic urticaria. However, acute urticaria was associated with a parental history of asthma, AR, and AD, remodeling of the house, and moving into another house, whereas chronic urticaria was associated with living in a new house, but was not associated with family history of allergic diseases.
A valid estimate of the prevalence and severity of urticaria can be determined by a survey of the general population, because most cases are treated by family doctors or over-the-counter medications.5 The prevalence of acute urticaria in our population (13.9%) was higher than previously reported in Western populations (3.4%-5.4%), possibly due to ethnic differences, demographic differences in the study populations,2425 performance of surveys at different time periods,7 disparities in the items included in the questionnaires,21 and differences in the external environments. In contrast to previous studies, our study included specific questionnaire items related to urticaria and is a community-based survey. Thus, the values reported in the present study represent the prevalence of urticaria in the general Korean pediatric population in an urban area. We found that acute urticaria is a highly prevalent disease in children, as are other allergic diseases, such as AD, AR, and asthma.
Previous studies have estimated the incidence of chronic urticaria in adults to be 0.5% to 5%. We found the prevalence of chronic urticaria to be 1.8% (i.e., chronic continuous urticaria, 0.7%; chronic recurrent urticaria, 1.1%) in children who were 4 to 12 year-old. Chronic recurrent urticaria726 is also known as chronic episodic urticaria based on the interval between episodes of symptom aggravation in chronic urticaria.32227 The present study is the first to report the prevalence of chronic continuous and chronic recurrent urticaria in children. However, we cannot compare our results with those of previous studies because there is no data on the prevalence of chronic urticaria with subdivision by continuous and recurrent types.
Previous researchers have estimated the prevalence of urticaria in pediatric populations indirectly based upon extrapolation from data on adults,7 by selecting chronic patients among all urticaria patients who visited a hospital,810 or by examination of a small number of children from the general population.2 There are no previous community-based studies of chronic urticaria in children. Previous studies have suggested that the prevalence of chronic urticaria in children was low compared to that in adults. However, we found similar prevalence rates of chronic urticaria in children and adults. More specifically, when we defined the chronic urticaria as chronic continuous urticaria, its prevalence was 0.7%, similar to the point prevalence previously reported for chronic urticaria in adults, 0.6% to 0.8%.26
We found that acute urticaria had a significantly higher risk of co-morbidity with other allergic diseases, such as asthma, AR, and AD, and with parental history of these allergic diseases. This finding is in good agreement with those of previous studies,121 and it is well known that acute urticaria is closely related to other allergic traits.3 A study by James et al.28 found a positive correlation between chronic urticaria and other allergic diseases, though another study reported a negative correlation.20 The latter study, whose results correspond well with our results, was an epidemiologic investigation comparable to the present study. In addition, we found no significant differences in total IgE, specific IgE, or total eosinophil counts between the control and the chronic urticaria groups, a finding in agreement with that of a previous study.29 These results support the interpretation that chronic urticaria does not have a significant relationship with systemic IgE-mediated reactions.
Our study showed no differences in the sex ratios of those with acute and chronic urticaria, in contrast to several studies of adults,236 but agreeing with a previous study of children.20 Our finding of a higher prevalence of chronic urticaria in boys was not statistically significant. The explanation for this finding could be similar to that previously proposed for other atopic diseases in children and adults.26
We found that the 25-hydroxyvitamin D level was not significantly different between the control and chronic urticaria groups, though there was no difference in age, sex or body mass index between the groups. However, recent studies reported that vitamin D levels in adults are inversely correlated with chronic urticaria,15 chronic urticaria severity,16 and treatment responsiveness of patients with chronic urticaria.18 The discrepancy between ours and previous studies may be due to: (1) differences between adults and children with regard to hormone secretion as well as the uptake and utility of calcium and vitamin D;30 (2) difference in control populations, healthy controls, and controls with allergic rhinitis;15 (3) differences in vitamin D levels (since the younger generation is at risk for vitamin insufficiency in Korea,31 vitamin D insufficiency may not have a significant difference on chronic urticaria as the other population of previous studies); (4) the time of blood sampling because of seasonal variation in vitamin D level differences (since the subjects were tested at a certain time of the year, the test results may not reflect the overall vitamin D levels throughout the whole year);1617 and (5) the number of patients with chronic urticaria is small in our study (it may not be the representative value).
We found that cold exposure significantly increased the prevalence of chronic urticaria but not acute urticaria, which is in agreement with a previous study of chronic urticaria in children.32 The BSACI guidelines also indicate that cold exposure is one of the most common precipitating factors for chronic urticaria.22 Thus, it may be that chronic urticaria is related to non-atopic AR or non-atopic triggering factors, but we cannot confirm this hypothesis.
Our finding that chronic urticaria was associated with more wheals than acute urticaria corresponds with a previous finding that disease severity is greater in subjects with chronic urticaria than acute urticaria.25 However, urticaria symptoms frequently change. Therefore, we measured the subject's maximum symptoms; this scoring system is different from the urticarial activity scoring system recommended by the EAACI/GA2LEN/EDF/WAO Guideline, which states that the sum of the scores from 7 consecutive days should be used in routine clinical practice to determine disease activity and response to treatment of chronic urticaria.23 For this reason, the disease severity of our patients cannot be directly compared with that of other studies.
Interestingly, we found that higher income was significantly associated with chronic continuous urticaria after adjusting for confounding factors that are well-known to be associated with AD (age, sex, family history of allergic condition, and past history).1 Previous research reported that socioeconomic status was associated19 or not associated220 with chronic urticaria. In addition, we found that living in a new house and environmental exposure were risk factors of urticaria. Further studies are needed to confirm the effect of indoor pollutant exposure on chronic urticaria in cases of remodeling house or moving into another house and to assess the relationships of environmental pollutants and indoor chemicals with symptoms of urticaria.33
The strengths of the current study include the large sample size and the use of a comprehensive and stringent questionnaire. We also examined risk factors related to environmental exposure and socioeconomic status. However, we could not establish cause-and-effect relationships and did not evaluate the underlying causes of chronic urticaria, which include autoreactivity, intolerance to food, physical urticaria (cholinergic urticaria and delayed pressure urticaria), dermographism, and infection.5 Thus, we did not confirm survey answers via telephone interviews. Although the reported prevalence of urticaria may depend on the diagnostic criteria, there is a reasonable concordance of prevalence estimates based on questionnaires and treatment or diagnostic history. Therefore, the prevalence obtained via survey questionnaires may be similar to that reported in clinics. However, the current study is limited due to recall bias with regard to the history of urticaria and lack of information on the use of antihistamines at the time of survey, which may have influenced the skin test results. Another limitation is that the number of subjects with chronic continuous urticaria was relatively small and therefore may not have been of representative value.
The present study is the first population-based assessment of the prevalence of chronic urticaria in children. The prevalence of chronic urticaria in children appears to be similar to that reported for adults. Chronic urticaria is influenced by potential irritants in the environment and socioeconomic status, but it is not influenced by the serum vitamin D levels, atopic status and past history, and parental history of allergic conditions. These results suggest that chronic continuous urticaria should be considered a disease distinct from acute urticaria. Further studies are required to confirm the causes of chronic urticaria and to delineate the underlying mechanisms.
References
1. Brüske I, Standl M, Weidinger S, Klümper C, Hoffmann B, Schaaf B, et al. Epidemiology of urticaria in infants and young children in Germany--results from the German LISAplus and GINIplus Birth Cohort Studies. Pediatr Allergy Immunol. 2014; 25:36–42. PMID: 24236825.
2. Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. 2010; 35:869–873. PMID: 20456386.

3. Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014; 133:1270–1277. PMID: 24766875.

5. Church MK, Weller K, Stock P, Maurer M. Chronic spontaneous urticaria in children: itching for insight. Pediatr Allergy Immunol. 2011; 22:1–8. PMID: 21261741.

6. Gaig P, Olona M, Muñoz Lejarazu D, Caballero MT, Domínguez FJ, Echechipia S, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004; 14:214–220.
8. Khakoo G, Sofianou-Katsoulis A, Perkin MR, Lack G. Clinical features and natural history of physical urticaria in children. Pediatr Allergy Immunol. 2008; 19:363–366. PMID: 18167159.

9. Hamel-Teillac D. Chronic urticaria in children. Ann Dermatol Venereol. 2003; 130 Spec No 1:1S69–1S72. PMID: 12843811.
10. Tuchinda M, Srimaruta N, Habanananda S, Vareenil J, Assatherawatts A. Urticaria in Thai children. Asian Pac J Allergy Immunol. 1986; 4:41–45. PMID: 2873823.
11. Konstantinou GN, Papadopoulos NG, Tavladaki T, Tsekoura T, Tsilimigaki A, Grattan CE. Childhood acute urticaria in northern and southern Europe shows a similar epidemiological pattern and significant meteorological influences. Pediatr Allergy Immunol. 2011; 22:36–42. PMID: 21261743.

12. Ehlers I, Niggemann B, Binder C, Zuberbier T. Role of nonallergic hypersensitivity reactions in children with chronic urticaria. Allergy. 1998; 53:1074–1077. PMID: 9860240.

13. Du Toit G, Prescott R, Lawrence P, Johar A, Brown G, Weinberg EG, et al. Autoantibodies to the high-affinity IgE receptor in children with chronic urticaria. Ann Allergy Asthma Immunol. 2006; 96:341–344. PMID: 16498857.

14. Kulthanan K, Jiamton S, Gorvanich T, Pinkaew S. Autologous serum skin test in chronic idiopathic urticaria: prevalence, correlation and clinical implications. Asian Pac J Allergy Immunol. 2006; 24:201–206. PMID: 17348242.
15. Thorp WA, Goldner W, Meza J, Poole JA. Reduced vitamin D levels in adult subjects with chronic urticaria. J Allergy Clin Immunol. 2010; 126:413. PMID: 20621341.

16. Woo YR, Jung KE, Koo DW, Lee JS. Vitamin D as a marker for disease severity in chronic urticaria and its possible role in pathogenesis. Ann Dermatol. 2015; 27:423–430. PMID: 26273159.

17. Oguz Topal I, Kocaturk E, Gungor S, Durmuscan M, Sucu V, Yıldırmak S. Does replacement of vitamin D reduce the symptom scores and improve quality of life in patients with chronic urticaria? J Dermatolog Treat. 2016; 27:163–166. PMID: 26295454.

18. Boonpiyathad T, Pradubpongsa P, Sangasapaviriya A. Vitamin D supplements improve urticaria symptoms and quality of life in chronic spontaneous urticaria patients: a prospective case-control study. Dermatoendocrinol. 2014; 6:e29727. PMID: 25346784.

19. Maurer M, Weller K, Bindslev-Jensen C, Giménez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy. 2011; 66:317–330. PMID: 21083565.
20. Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergológica 2005. J Investig Allergol Clin Immunol. 2009; 19(Suppl 2):21–26.
21. Kjaer HF, Eller E, Høst A, Andersen KE, Bindslev-Jensen C. The prevalence of allergic diseases in an unselected group of 6-year-old children. The DARC birth cohort study. Pediatr Allergy Immunol. 2008; 19:737–745. PMID: 18318699.

22. Powell RJ, Du Toit GL, Siddique N, Leech SC, Dixon TA, Clark AT, et al. BSACI guidelines for the management of chronic urticaria and angio-oedema. Clin Exp Allergy. 2007; 37:631–650. PMID: 17456211.

23. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009; 64:1417–1426. PMID: 19772512.
24. Mortureux P, Léauté-Labrèze C, Legrain-Lifermann V, Lamireau T, Sarlangue J, Taïeb A. Acute urticaria in infancy and early childhood: a prospective study. Arch Dermatol. 1998; 134:319–323. PMID: 9521030.
25. Sackesen C, Sekerel BE, Orhan F, Kocabas CN, Tuncer A, Adalioglu G. The etiology of different forms of urticaria in childhood. Pediatr Dermatol. 2004; 21:102–108. PMID: 15078346.

26. Zuberbier T, Greaves MW, Juhlin L, Kobza-Black A, Maurer D, Stingl G, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001; 6:123–127.
27. Grattan CE, Humphreys F. British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007; 157:1116–1123. PMID: 18021095.

28. Zazzali JL, Broder MS, Chang E, Chiu MW, Hogan DJ. Cost, utilization, and patterns of medication use associated with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012; 108:98–102. PMID: 22289728.

29. Chang KL, Yang YH, Yu HH, Lee JH, Wang LC, Chiang BL. Analysis of serum total IgE, specific IgE and eosinophils in children with acute and chronic urticaria. J Microbiol Immunol Infect. 2013; 46:53–58. PMID: 22560476.

30. Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002; 30:771–777. PMID: 11996918.

31. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011; 96:643–651. PMID: 21190984.

32. Visitsunthorn N, Tuchinda M, Vichyanond P. Cold urticaria in Thai children: comparison between cyproheptadine and ketotifen in the treatment. Asian Pac J Allergy Immunol. 1995; 13:29–35. PMID: 7488341.
33. Park DW, Kim SH, Moon JY, Song JS, Choi J, Kwak HJ, et al. The effect of low-VOC, water-based paint on aggravation of allergic disease in school children. Indoor Air. 2016; Forthcoming.
Fig. 1
Enrolment and classification of children (4 to 13 year-old) who reported having had wheals or angioedema at some time during their lifetimes (n=917, 22.5%) and controls (n=3,159, 77.5%).
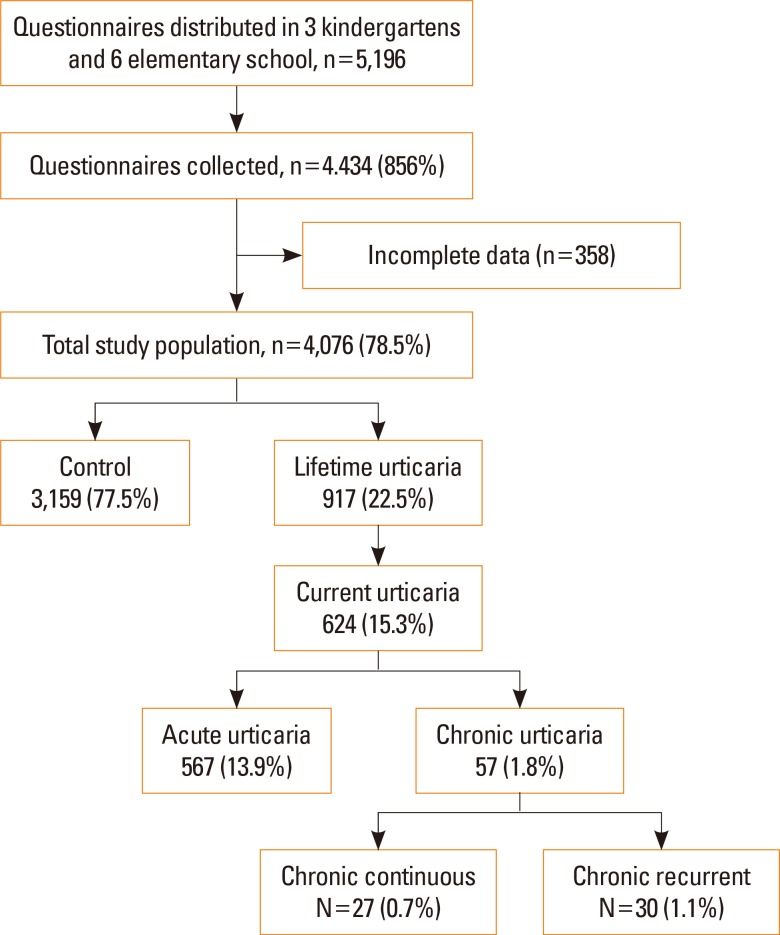
Fig. 2
Urticaria activity scores (UAS; mild, moderate, and intense) of subjects with chronic and acute urticaria. The maximum UAS for wheals was significantly higher for individuals with chronic urticaria than for those with acute urticaria (1.7±0.8 vs 1.3±0.7, P=0.007), but there was no significant difference for pruritus (acute: 2.6±0.9, chronic: 2.7±1.1, P=0.515).
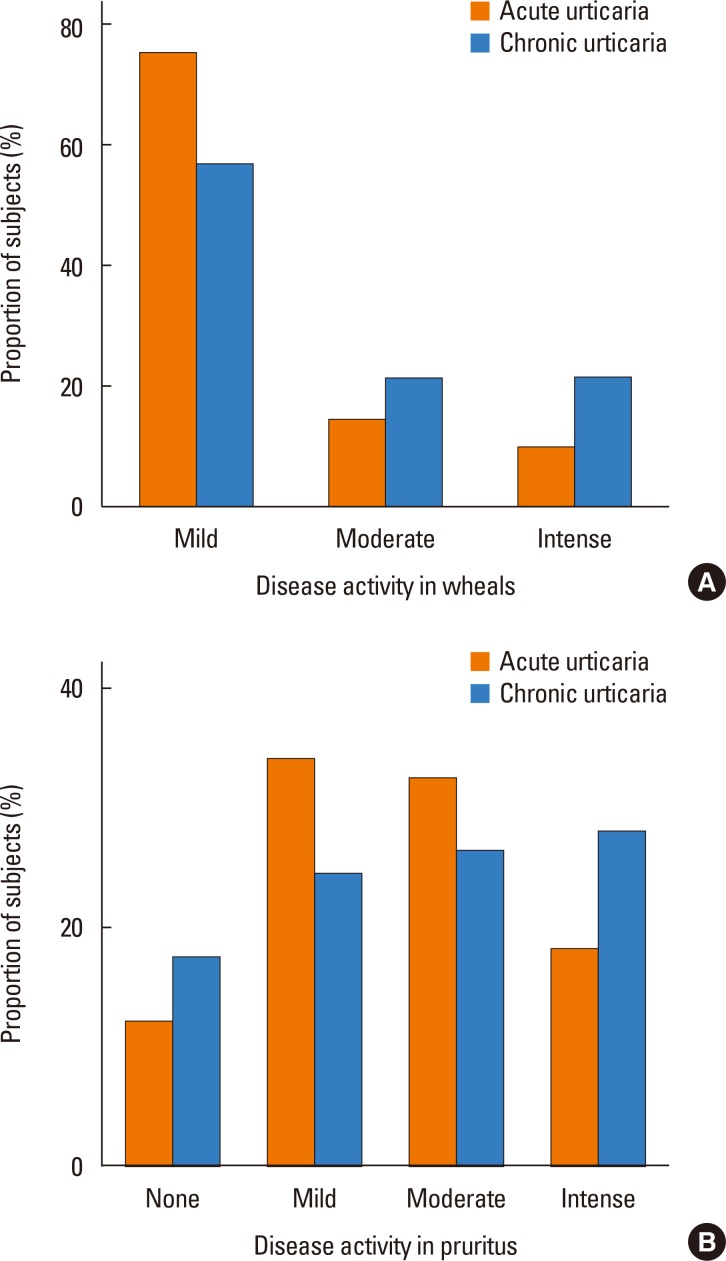
Fig. 3
Causes of acute and chronic urticaria. The most common tentative causes were food (n=236, 37.8%) and changing environment (n=172, 27.6%). Cold exposure was the only factor significantly different for subjects with chronic and acute urticaria (14% vs 6.7%, P=0.043).

Table 1
Demographic characteristics of the study population (N=4,076)
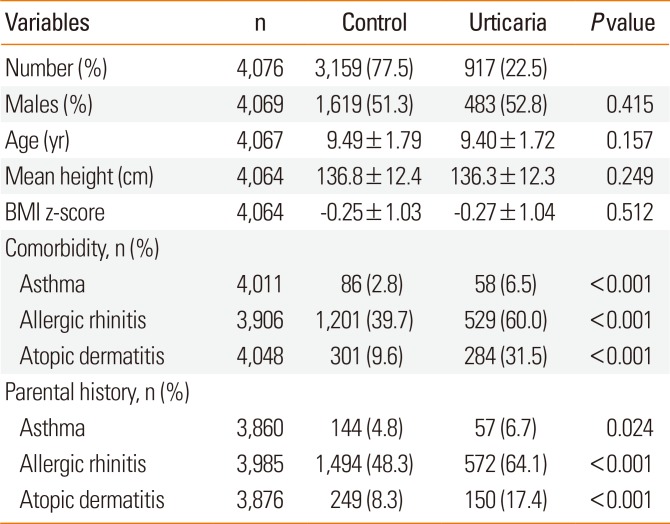
Table 2
Demographic and clinical characteristics of subjects with acute and chronic urticaria (n=624)
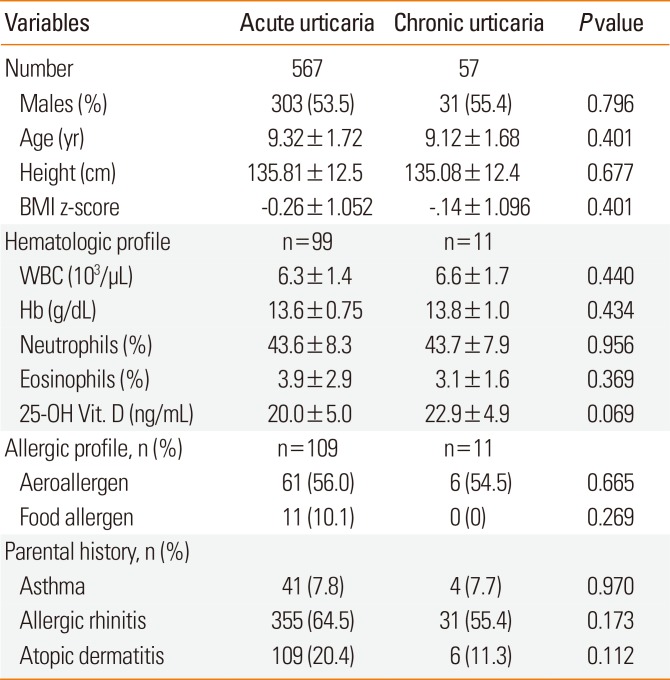
Table 3
Adjusted odds ratios* of acute urticaria and chronic urticaria relative to controls
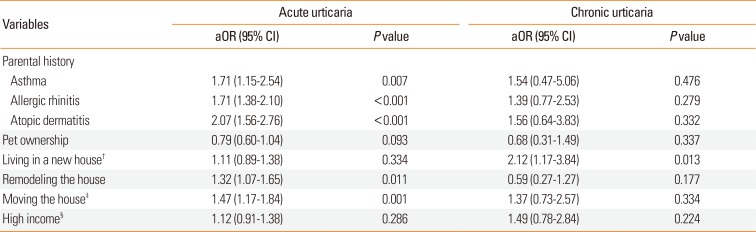
Table 4
Adjusted odds ratios* of chronic recurrent urticaria and chronic continuous urticaria relative to controls
aORs, adjusted odds ratios; CI, confidence interval.
*Adjustment for age, sex, BMI z-score, personal history of atopic dermatitis, and parental history of allergic conditions (i.e., asthma, allergic rhinitis, and atopic dermatitis) was used to identify independent risk factors for acute and chronic urticaria (continuous type and recurrent type); †Living in a new house was defined as age of the residence less than 6 years; ‡Moving the house fewer than 12 months after birth; §Total household income over $3,360 per month.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download