Abstract
Purpose
Atopic dermatitis (AD) is a chronic eczematous dermatitis that has a high prevalence and diverse clinical features. Although several hypotheses about its multifactorial pathogenesis have been suggested, the cause is not yet fully understood. A better understanding of the clinical features may helpful inelucidating the pathogenesis of AD.
Methods
This retrospective study analyzed the questionnaires, medical charts, and laboratory examination results of 5,000 patients diagnosed with AD at a single tertiary hospital in Korea.
Results
The demographics, allergic comorbidities, family history, severity, and treatment experiences of the patients were analyzed. Most of the patients were adults, 76.3% of whom were classified as havingan extrinsic type of AD. The mean eczema area and severity index (EASI) score was found to be 13.68, and adult patients were found to have higher severity than the other age groups. The anatomical involvements were different among the age groups, with more involvements of the head and neck in adults. The patients reported seasonal changes and stress as the factors that aggravated their symptoms the most. Topical steroids and oral cyclosporine were the most used medications at our clinic, whereas 10.1% of the patients underwent allergen-specific immunotherapy.
Atopic dermatitis (AD) is a chronic relapsing, severely pruritic, eczematous skin disease that may be accompanied by other allergic diseases, such as asthma and allergic rhinitis. The incidence of AD has steadily increased, with an estimated prevalence of 15%-0% in children and 1%-3% in adults.12 The symptoms of AD may appear in as early as infancy, which markedly improve or clear before or during adolescence in about 30%-50% of the patients. The persistence of AD until adulthood is increasing, reaching up to 60%, and shows a more chronic and recurrent course, causing a significant social burden.3
Clinical features of AD are diverse, and this heterogeneity may be due to the multifactorial pathogenesis of the disease, in which defects of the skin barrier, environmental factors, and immunological dysregulation are involved.45 Immune dysregulation of cytokine imbalance with increased Th2 and Th22 skewing has been emphasized as a crucial pathogenesis that results in the disruption of keratinocytes.6 The primary disruption of the skin barrier, on the other hand, due to filaggrin mutation is also considered significant, allowing increased penetration of allergens and microbes, enhancing Th2 polarization-through dendritic cells. Due to such complicated mechanisms of pathogenesis, clinical features of patients with AD are complex and may differ even within the same age group.7 This was a prospectively designed, retrospectively analyzed study, in which we reviewed various clinical features and different subtypes of AD to better understand the clinical diversity of AD in Korea and help physicians treat their patients.
We reviewed the medical records and laboratory data of 5,000 patients who initially visited the outpatient clinic at the department of dermatology and was diagnosed with AD between October 2007 and January 2015. The diagnosis of AD was made according to the diagnostic features of Korean diagnostic criteria for AD,9 which were established on the basis on the guidelines of Hanifin and Rajka.8 The symptom severity was assessed by using the eczema area and severity index (EASI) score at the initial visit.10 Mild severity was defined as an EASI score of <7, moderate severity as ≥7 and <21, and severe AD as ≥21.11 The patients were divided into those with intrinsic and extrinsic types of AD. The intrinsic type was defined as a total IgE of <150 kU/L and no sensitization to allergens as confirmed by ImmunoCAPassay (Phadia, Uppsala, Sweden), whereas the extrinsic type was defined as a total IgE of >150 kU/L or positive sensitization in the CAP immunoassay.1213 The total IgE levels were assessed, in which the maximum measurable value was 5,000 kU/L. Thus, patients with higher values were regarded as having 5,000 kU/L.
At their initial visits, the patients were given a questionnaire that included information about the onset of AD; their medical histories of atopic diseases including allergic rhinitis, allergic conjunctivitis, and allergic asthma; family histories of atopic diseases; the factors that aggravated their symptoms; and the past treatments that they had received. For the aggravating factors, a specific list was given, which included seasonal variations, sweating, stress, food, and clothing (the entire list can be seen in Results).
At initial visits, blood tests were performed including complete blood count; routine chemistry; serum total IgE; and sensitization to specific allergens related to AD which include house dust mites (Dermatophagoidesfarina [D. farinae], Dermatophagoidespteronyssinus [D. pteronyssinus]), egg white, milk, flour, peanut, bean, pork, and cockroach, as detected with CAP immunoassay. Patients were considered sensitized to the allergens when the results of CAP immunoassay were greater than or equal to class 3 (≥3.5 kU/L).
Data are presented as mean±standard deviation (SD). The results were analyzed by using Student's t tests, 1-way analysis of variance, and χ2 tests. Deviations were considered statistically significant when P<0.05. SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Table 1 shows the demographics of the patients. There was no difference in the male-to-female ratio (M/F, 52:48), and the mean age of the patients was 18.99±10.22 years at the initial visit. Their ages ranged from early infancy to 86 years, with a predominance of adult patients >19 years old (51.1%). Most of the patients had an onset age of <18 years; those with onset during infancy (≤2 years old) was 28.0%, and those with onset during childhood and adolescence were 49.0%. A relatively high percentage (22.0%) of the patients developed AD after adulthood. The geographic data of the patients were also analyzed; most of the patients were from Seoul (57.1%), the capital of Korea,where our hospital is located. The patients came from all areas of Korea.Of the 5,000 patients, 0.4% came from Ulsan and 26.7% came from Gyeonggiprovince.
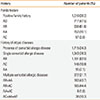
Allergic march is a feature ofAD, and the presence of allergic comorbidities, which include allergic rhinitis, allergic asthma, and allergic conjunctivitis, was assessed (Table 2). In our data, 34.3% of the AD patients had allergic comorbidities, with 78.3% having a single comorbidityand the rest (21.7%) having multiple comorbid atopic diseases. Of the patients with a single allergic comorbidity, allergic rhinitis was the most common (82.0%), followed by allergic conjunctivitis (10.9%) and allergic asthma (7.1%). The proportion of patients with any family history of atopy was 30.2%, with allergic rhinitis (61.6%) also being the most common, followed by AD (47.5%), allergic asthma (10.1%), and allergic conjunctivitis (3.8%).
Fig. 1 shows that 3,815 (76.3%) of the patients were classified as the extrinsic type, whereas the rest (1,185,23.7%) were classified as the intrinsic type. The 2 types were further categorized by age, and it was found that there were almost equal numbers of patients in infancy with the extrinsic vs intrinsic type (50.8% vs 49.2%) and that as the age increased, the proportion of patients with the extrinsic type, who are more sensitized to allergens, gradually increased up to 80.5% of adult AD patients. Sensitization to specific allergens was detected by using CAP immunoassay (Fig. 2), and of the several allergens tested, D. farinae (59.7%) and D. pteronyssinus (59.5%) were found to have the highest sensitization rates. The level of total IgE was increased in most of the patients, with an average of 1,319.35±1,609.77 kU/L.
The percentage of each feature fulfilled in the Korean diagnostic criteria for AD was assessed. Of the major features, pruritus was observed in all the patients; of the minor features, xerosis was most common, followed by pruritus with sweating (Fig. 3).
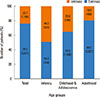
The affected areas of the body were evaluated (Fig. 4), and symptoms that were dispersed throughout the whole body were found in 46% of the patients. The head and neck area was involved in 35% of the patients, followed by the upper extremities (33%), lower extremities (25%), and trunk (16%). These data differ among different age groups: infants and adults showed involvements in their head and neck area the most (25.3% and 23.9%, respectfully), whereasin patients in their childhood (ages 2-12 years),the upper extremities were the most involved body part (22.6%). The proportion of different clinical subtypes that presented with AD was also assessed.Patients who accompanied the nummular eczema type were found to be 7.65%, followed by those with the prurigo type (2.78%), red face (1.15%), and lichen amyloidosis (0.51%). In addition, 5.61% of the patients were found to have eczema herpeticum.
To assess the severity of AD, the EASI score was used, and the average EASI score was found to be 13.68 (±10.22). The proportion of patients with mild severity was 28.2%, those with moderate severity 42.8%, and those with severe severity 29.0%. Further stratifying the EASI score according to different age groups, patients in their infancy (age≤2 years) were mostly found to have either mild or moderate symptoms (90.4%, EASI<21), and as the age of the patients increased, the severity score increased, with 34.10% of the patients in their adolescence or adulthood classified as having severe AD (Fig. 5A). In addition, patients who had AD that persisted until adulthood and those with pure adult-onset AD were compared (Fig. 5B). Adult patients with AD onset before adulthood had an average EASI score of 17.04 (±13.93), whereas those with adult-onset AD had a score of 14.59 (±12.41), and the difference was statistically significant (P<0.01).
The EASI scores were also compared between male and female patients (Fig. 6A). The mean EASI score was 16.47 (±12.66) in male patients and 12.69 (±11.11) in female patients, and the difference was statistically significant (P<0.001). The relationship between sex and severity was further stratified and analyzed (Table 3). In the group with mild severity, 40.4% were male patients and 59.6% were female patients. The proportion of male patients increased with increased severity. For moderate severity, the proportions of male and female patients were 53.9% and 46.1%, respectively, and there were 62.2% males and 37.8% females in the severe group. The differences in the prevalence of the patients of different sexes between individual severity groups were statistically significant (P<0.001). The relationship between the total IgE levels and EASI scores were assessed (Fig. 6B). The mean total IgE level of the group with mild severity was 694.08 kU/L. The mean total IgE levels of the moderate and severe groups were 1,319.81 and 2,612.27 kU/L, respectively, and the differences between the 3 groups were statistically significant (P<0.001).
Aggravating factors were assessed by using a survey (Fig. 7A). For the 25 factors surveyed, 3,951 (79%) patients selected seasonal changes as the aggravating factor that affected their symptoms the most, followed by stress (2,998, 60%), dryness (2,550, 51%), and sweating (1,847, 37%). For months during which the symptoms were most severe were also assessed (Fig. 7B), 2,335 and 2,421 patients responded that as the months when their symptoms were worst July and August (46.7% and 48.4%, respectively), followed by December (2,208, 44.2%) and January (1,785, 35.7%). The month during which the symptoms were least severe was May, as reported by 821 (16.4%) patients.
The patients were also questioned on all treatment methods that had been used before their visit to our clinic. Diverse treatments have been attempted, and topical steroids were the most commonly used treatment (70.9%) added on moisturizers used by 85.8% of the patients. Of the patients, 46.7% reported trying folk remedies (Fig. 8A).
At our clinic, the most frequently prescribed medication was topical steroids (60.9%), followed by low-dose oral cyclosporine (56.7%). Oral steroids were not commonly used (25.8%) and only prescribed during acute exacerbations. Topical calcineurin inhibitors were used in 17.5% of the patients, and allergen-specific immunotherapy was done in 10.1% of the patients (Fig. 8B).
AD has been known to have no sex difference in prevalence until the age of 6 years; thereafter, the number of female patients becomes slightly higher than that of male patients.1 In our study, no difference in prevalence was found between the sexes (M/F, 52:48). The percentage of patients aged >18 years at the initial visit was 51.1%, with a mean age of 18.99 years. Compared with previous epidemiologic studies of AD, this relatively older age group can be attributed to the fact that the data were obtained from a clinic in a tertiary hospital, where patients with a more chronic course that persists until adulthood would be more likely to visit. In terms of geographic data, although most of the patients were from Seoul (57.1%), the capital city and also where our hospital is located, the remaining 42.9% of the patients were from all the other cities. The higher number of patients from Seoul may be due to the location of our hospital, but the fact that the population is higher in larger urban cities may have contributed to the results.14
A positive family history of allergic diseases was found in 30.2% of the patients in our study. Of the allergic diseases, allergic rhinitis was the common (61.6%), followed by AD (47.5%). Family history, one of the major criteria in the diagnosis of AD, is known to be an important risk factor for AD. Studies from Western countries have found a positive family history of atopy in 53%-80% of patients.1516 In a recent study in Korean children with AD, about 40% had a family history,17 whereas a few others have shown a positive family history in 28% and 47.8%.1518 In a study on adult-onset AD, 54.2% had a family history with most cases having allergic rhinitis.19 Patients with a family history of atopy were fewer in our study than in previous studies. However, the results are diverse, and further studies are needed to determine whether this discrepancy is due to different ethnicities, age groups, or other undetermined factors.
CAP immunoassay was performed to evaluate the presence of sensitization to specific allergens, and D. farinae (59.7%), and D. pteronyssinus (59.5%) were found to be the allergens to which most patients were sensitized. House dust mites have been considered the major allergen related to AD, and the proportion of sensitization to house dust mites in AD patients was similar to those reported by previous studies.2021 In several studies, efforts have been made in reducing house dust mites; yet, no significant progress has been seen.22 In a recent study, D. pteronyssinus was found to be a major allergen for house dust mite-allergic AD patients, unlike in those with respiratory allergy, suggesting the possibility of a useful serological marker.23 Although more prominent in extrinsic types of AD, the total IgE levels were generally increased, with an average of 1,319.35±1,609.77 kU/L found in our patients. In many previous reports, specific IgE or total serum IgE levels have been found to be significantly associated with the severity of AD.2425 Based on of these findings, measurement of the total IgE level is significant in patients with AD.
AD may be classified into 2 different types, extrinsic and intrinsic, which are also called allergic and non-allergic types. The intrinsic type, or non-allergic type, shows normal IgE levels in addition to the absence of any sensitization to allergens, whereas the extrinsic or allergic type has increased specific IgE levels with sensitization to specific allergens.13,26 Of patients with AD, 76.3% were found to havethe extrinsic types,whereas the rest (23.7%) had the intrinsic type. Our data seemed consistent with those of previous reports: the incidence of extrinsic vs intrinsic AD in Germany (63% vs 37%),27 in Netherlands (78.2% vs 21.8%),28 and in Korea (80% vs 20%).29 Comparing the 2 types among different age groups, the proportion was similar between the extrinsic vs intrinsic type in the infancy group (age ≤2 years, 50.8% vs 49.2%), whereas the number of patients with the extrinsic type increased with age, reaching 80.5% in the adolescent and adult groups (age >11 years). In previous reports, the intrinsic type was found to have a higher frequency in children than inadults,30 with a 57% of intrinsic AD cases in infants31 and an 87.5% of extrinsic AD cases in adults,26 which are similar to our data. Therefore, sensitization to allergens increases with age, leading to the increase in the proportion of the intrinsic types of AD. Moreover, our data are meaningful as they are consistent with those of previous studies and have been confirmed in >5,000 patients.
Most of the patients had generalized eczematous lesions. The head and neck area was involved in 35% of the patients, followed by the upper extremities (33%), lower extremities (25%), and trunk (16%). Patients in their infancy had the highest tendency to have symptoms on the head and neck, whereas older children had more symptoms on the extremities, specifically in the flexural areas. The adults also had symptoms most commonly on the head and neck, followed by the upper extremities that included eczemas on the hands, being consistent with the result of previous studies.32 In our study, significantly lower vitamin D levels were found in patients with AD, which was also most significantly related to eczema of the head and neck.33 Further investigations are needed into the involvement of different anatomical sites according to different age groups, in which vitamin D levels may be one of theassociated factors.
In our study, the severity of AD was mainly assessed with the EASI score, and the average EASI score of our patients was 13.68 (±10.22), corresponding to the moderate severity. Most of the infants had mild to moderate severity; however, the EASI score increased with age, reaching >30% of that of severe adult patients. As in previous studies, the severity was either mild or moderate in >80% of the infants and children,1518 whereas a higher percentage (25%-31%) of patients in their adulthood had severe AD.734 Some other studies, however, showed contradictory data, with only 1.7% of adult patients having severe AD.19 We also analyzed whether the disease severity differed between the adults with onset before adulthood and those with onset after adulthood. Those with earlier onsets had a statistically higher severity, which may indicate that those with symptoms persisting until adulthood may have more severe symptoms.
Further analyses concerning the severity of AD were performed, and the association of sex with severity showed that male patients had significantly higher EASI scores than female patients. A previous study in the Korean population found a higher prevalence of male patients than in female patients.35 Based on this study, the prevalence in each sex was additionally analyzed by further subdividing the sex groups according to severity. As a result, more female patients belonged to the mild group, and the proportion of male patients increased with increasing severity, with significant differences between sexes. Furthermore, the total IgE levels were assessed to determine their correlation with severity. The 3 groups stratified according to severity by using EASI scores showed that the increased EASI scores correlated with the total IgE levels. A study of 345 children found that serum IgE levels correlated with the severity of AD as assessed according to the severity scoring of AD or the severity scoring of atopic dermatitis (SCORAD) index.36 Our study also proved the correlation of serum IgE levels with the severity of AD, which is a meaningful result as a large number of patients were analyzed.
Several morphological variants can be present in patients with AD, with the nummular eczema type being the most common (7.65%) in patients at our clinic. The onset of nummular eczema has been known to be mostly during adulthood, between ages 20 and 60 years, but it is also common in childhood.37 Its pathogenesis and relationship to AD is unclear; however, it appears to be the most common atypical type of AD, which represents almost about 50% of all types,719 consistent with the data from our patients. Prurigonodularis is also another variant that can be observed in patients with AD, and 2.78% of the patients presented with the prurigo type. Although AD is a major cause, the mechanism of prurigo nodularis is unclear and the treatment may be challenging.38
Eczema herpeticum, or disseminated vesicular eruption caused by herpes simplex virus, was found in 5.61% of the patients. Because of the disruption of the skin barrier, AD patients are more susceptible to cutaneous viral infections, including eczema herpeticum. It affects approximately 3% of AD patients and may be fatal, causing keratoconjunctivitis, meningitis, and viremia, if untreated.39 Early onset, higher disease severity, greater Th2 polarization, impaired skin barrier, and sensitization to multiple allergens are some factors associated with increased susceptibility to eczema herpeticum.40 The higher percentage of patients with eczema herpeticum in our study may be because our facility is a tertiary hospital that is visited by patients with higher severity.
For numerous environmental factors that may affect the symptoms of AD, patients who visited our facility reported that seasonal changes, stress, dryness, and sweating were the factors aggravating their symptoms the most. This result probably contributes to the fact that AD patients are sensitive to environmental conditions, and direct irritation to the skin, such as that caused by sweating or dryness, can easily aggravate the symptoms of AD. The patients reported that August (48%) is the month when their symptoms were worst, followed by July (47%), December (44%), and January and November (36%). In a previous study in the United Kingdom, hot weather and sweating were found to be the most common exacerbating factors,41 which can explain why the symptoms worsen during summer. In another study in Norway, however, the symptoms of AD improved after moving to a warm subtropical climate from a subarctic climate, which was explained by the effects of ultraviolet (UV) radiation from increased sunexposure.42 The same result was also observed in a study done in Italy, which can also be explained by the effects of increased sun exposure. The discrepancy in aggravation between summer or winter may be caused by the latitude and differences in the temperature, humidity, and climate of different regions.43 Likewise, summers in Korea are excessively humid with high temperatures, which may lead to increased stress and thus aggravation of symptoms.
Patients with AD are frequently sensitized to house dust mites, and several recent, reports have shown that allergen-specific immunotherapy is a safe and efficacious treatment.444546 At our facility, allergen-specific immunotherapy is recommended to patients with the extrinsic type of AD showing severe symptoms and experiencing frequent flare-ups, to those unresponsive to conventional treatments, and to those who were able to regularly follow the schedules. Of the patients, 10.1% were on immunotherapy for at least 1 year unless remission was achieved. Immunotherapy was maintained for more than 3 years in responsive patients. Sensitization to house dust mites was confirmed in these patients, and allergen-specific immunotherapy was proved to be effective, with 92.7% of the patients showing improvement of pruritus and 45% of the patients achieving remission. Only 16% of the patients showed either minimal improvement or no change.4447 Its safety was also proved, with only 0.1% of systemic adverse effects, such as flu-like symptoms, observed in our institution. No fatal adverse events were reported, and other adverse effects were minimal, including urticaria or pruritus, and only seen in <1% of the patients.48 Although the need for frequent visit and long treatment time may discourage patients to receive the therapy and lead to low compliance,49 allergen-specific immunotherapy can be a useful option for refractory patients sensitized to house dust mites.
In the past, AD was generally considered a disease confined to infant-children-adolescent age groups. Recently, however, AD that progresses until adulthood and adult-onset AD have been steadily increasing. Most of the AD patients who had visited our clinic were adults,and we have demonstrated that these patientshave more severe symptoms, different anatomical distributions with increased involvement of the head and neck, and a higher proportion of the extrinsic type. Considering various subtypes with diverse clinical features and different manifestations among different age groups of AD, treatment strategies should be differentiated according to the age groups and subtypes in order to effectively treat patients.48
Figures and Tables
 | Fig. 1The proportion of extrinsic and intrinsic types according to different age groups. The age groups were defined as follows: infancy ≤2 years old, childhood and adolescence >2 years old and ≤18 years old, and adulthood>18 years old. The intrinsic type was defined as a total IgE of <150 kU/L and with no sensitization to allergens, whereas the extrinsic type was defined as a total IgE of >150 kU/L or a positive sensitization in CAP immunoassay. |
 | Fig. 2Evaluation of sensitization to specific allergens in CAP immunoassay. D. pteronyssinus, Dermatophagoidespteronyssinus; D. farinae, Dermatophagoidesfarinae. |
 | Fig. 3The percentages of each fulfilled criterion of the (A) major features and (B) minor features of the diagnostic criteria. PHx, past history; FHx, family history. |
 | Fig. 4Proportion of the involved parts of the body according to different age groups.The age groups are defined as follows: infancy ≤2 years old, childhood and adolescence >2 years old and ≤18 years old, adulthood >18 years old. |
 | Fig. 5Association of severity and age. (A) The patients of different age groups stratified by EASI scores into mild, moderate, and severe groups. (B) The differences of EASI scores between adult patients with onset before adulthood and those with adultonset. The age groups were defined as follows: infancy ≤2 years old, childhood and adolescence >2 years old and ≤18 years old, and adulthood >18 years old.The EASI scores ranged from 0 to 72, with the scores stratified as follows: mild <7, moderate ≥7 and <21, and severe ≥21. EASI, eczema area and severity index. *P<0.01. |
 | Fig. 6(A) Comparison of EASI scores in male and female patients.(B)The association of totalIgE levels of different severity groups categorized by EASI scores. The EASI scores ranged from 0 to 72. The severity was stratified according to EASI scores as follows: mild <7, moderate ≥7 and<21, and severe ≥21. EASI, eczema area and severity index. *P<0.001. |
 | Fig. 7(A) Aggravating factors. (B) Aggravation of symptoms according to months of the year assessed by using a questionnaire. |
Table 1
Number of patients (%) by sex, age at the initial visit, and age of onset
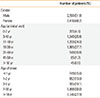
Table 2
Number of patients with a family history of allergic diseases and comorbid atopic diseases
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI11C1671, HI13C0010, HI14C1799, HI14C1324) and a faculty research grant of Yonsei University College of Medicine (6-2010-0117).
References
1. DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc. 2012; 33:227–234.
2. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015; 66:Suppl 1. 8–16.
3. Kim C, Park KY, Ahn S, Kim DH, Li K, Kim DW, et al. Economic impact of atopic dermatitis in Korean patients. Ann Dermatol. 2015; 27:298–305.
4. Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015; 45:566–574.
5. Novak N, Leung DY. Advances in atopic dermatitis. Curr Opin Immunol. 2011; 23:778–783.
6. Malajian D, Guttman-Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine. 2015; 73:311–318.
7. Pugliarello S, Cozzi A, Gisondi P, Girolomoni G. Phenotypes of atopic dermatitis. J Dtsch Dermatol Ges. 2011; 9:12–20.
8. Hanifin JM, Rajka G. Diagnostic features of atopic eczema. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
9. Park YL, Kim HD, Kim KH, Kim MN, Kim JW, Ro YS, et al. Report from ADRG: a study on the diagnostic criteria of Korean atopic dermatitis. Korean J Dermatol. 2006; 44:659–663.
10. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001; 10:11–18.
11. Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015; 172:1353–1357.
12. Schmid-Grendelmeier P, Simon D, Simon HU, Akdis CA, Wüthrich B. Epidemiology, clinical features, and immunology of the “intrinsic” (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis). Allergy. 2001; 56:841–849.
13. Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010; 58:1–7.
14. Lee JH, Han KD, Kim KM, Park YG, Lee JY, Park YM. Prevalence of atopic dermatitis in Korean children based on data from the 2008–2011 Korean National Health and Nutrition Examination Survey. Allergy Asthma Immunol Res. 2016; 8:79–83.
15. Wananukul S, Chatproedprai S, Tempark T, Phuthongkamt W, Chatchatee P. The natural course of childhood atopic dermatitis: a retrospective cohort study. Asian Pac J Allergy Immunol. 2015; 33:161–168.
16. Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015; 7:101–105.
17. Chung Y, Kwon JH, Kim J, Han Y, Lee SI, Ahn K. Retrospective analysis of the natural history of atopic dermatitis occurring in the first year of life in Korean children. J Korean Med Sci. 2012; 27:723–728.
18. Cosickic A, Skokic F, Colic-Hadzic B, Jahic M. Clinical characteristics and estimation severity of the atopic dermatitis in children. Med Arh. 2010; 64:178–182.
19. Kulthanan K, Samutrapong P, Jiamton S, Tuchinda P. Adult-onset atopic dermatitis: a cross-sectional study of natural history and clinical manifestation. Asian Pac J Allergy Immunol. 2007; 25:207–214.
20. Čelakovská J, Ettlerová K, Ettler K, Vaněčková J, Bukač J. Sensitization to aeroallergens in atopic dermatitis patients: association with concomitant allergic diseases. J Eur Acad Dermatol Venereol. 2015; 29:1500–1505.
21. Darsow U, Laifaoui J, Kerschenlohr K, Wollenberg A, Przybilla B, Wüthrich B, et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy. 2004; 59:1318–1325.
22. Garritsen FM, ter Haar NM, Spuls PI. House dust mite reduction in the management of atopic dermatitis.A critically appraised topic. Br J Dermatol. 2013; 168:688–691.
23. Banerjee S, Resch Y, Chen KW, Swoboda I, Focke-Tejkl M, Blatt K, et al. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J Invest Dermatol. 2015; 135:102–109.
24. Kiiski V, Karlsson O, Remitz A, Reitamo S. High serum total IgE predicts poor long-term outcome in atopic dermatitis. Acta Derm Venereol. 2015; 95:943–947.
25. Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis? J Allergy Clin Immunol. 2004; 114:150–158.
26. Kulthanan K, Boochangkool K, Tuchinda P, Chularojanamontri L. Clinical features of the extrinsic and intrinsic types of adult-onset atopic dermatitis. Asia Pac Allergy. 2011; 1:80–86.
27. Ott H, Stanzel S, Ocklenburg C, Merk HF, Baron JM, Lehmann S. Total serum IgE as a parameter to differentiate between intrinsic and extrinsic atopic dermatitis in children. Acta Derm Venereol. 2009; 89:257–261.
28. Brenninkmeijer EE, Spuls PI, Legierse CM, Lindeboom R, Smitt JH, Bos JD. Clinical differences between atopic and atopiform dermatitis. J Am Acad Dermatol. 2008; 58:407–414.
29. Choi SJ, Song MG, Sung WT, Lee DY, Lee JH, Lee ES, et al. Comparison of transepidermal water loss, capacitance and pH values in the skin between intrinsic and extrinsic atopic dermatitis patients. J Korean Med Sci. 2003; 18:93–96.
30. Fölster-Holst R, Pape M, Buss YL, Christophers E, Weichenthal M. Low prevalence of the intrinsic form of atopic dermatitis among adult patients. Allergy. 2006; 61:629–632.
31. Park JH, Choi YL, Namkung JH, Kim WS, Lee JH, Park HJ, et al. Characteristics of extrinsic vs intrinsic atopic dermatitis in infancy: correlations with laboratory variables. Br J Dermatol. 2006; 155:778–783.
32. Kanwar AJ, Narang T. Adult onset atopic dermatitis: Under-recognized or under-reported? Indian Dermatol Online J. 2013; 4:167–171.
33. Noh S, Park CO, Bae JM, Lee J, Shin JU, Hong CS, et al. Lower vitamin D status is closely correlated with eczema of the head and neck. J Allergy Clin Immunol. 2014; 133:1767–1770.e6.
34. Orfali RL, Shimizu MM, Takaoka R, Zaniboni MC, Ishizaki AS, Costa AA, et al. Atopic dermatitis in adults: clinical and epidemiological considerations. Rev Assoc Med Bras (1992). 2013; 59:270–275.
35. Kim MJ, Kang TW, Cho EA, Kim HS, Min JA, Park H, et al. Prevalence of atopic dermatitis among Korean adults visiting health service center of the Catholic Medical Center in Seoul Metropolitan Area, Korea. J Korean Med Sci. 2010; 25:1828–1830.
36. Laske N, Niggemann B. Does the severity of atopic dermatitis correlate with serum IgElevels? Pediatr Allergy Immunol. 2004; 15:86–88.
37. Cowan MA. Nummular eczema. A review, follow-up and analysis of a series of 325 cases. Acta Derm Venereol. 1961; 41:453–460.
38. Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013; 27:550–557.
39. Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013; 98:153–157.
40. Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy ClinImmunol. 2009; 124:260–269. 269.e1–269.e7.
41. Williams JR, Burr ML, Williams HC. Factors influencing atopic dermatitis-a questionnaire survey of schoolchildren's perceptions. Br J Dermatol. 2004; 150:1154–1161.
42. Byremo G, Rød G, Carlsen KH. Effect of climatic change in children with atopic eczema. Allergy. 2006; 61:1403–1410.
43. Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016; 30:223–249.
44. Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015; 7:221–229.
45. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013; 132:110–117.
46. Nahm DH, Kim ME, Kwon B, Cho SM, Ahn A. Clinical Efficacy of Subcutaneous Allergen Immunotherapy in Patients with Atopic Dermatitis. Yonsei Med J. 2016; 57:1420–1426.
47. Lee J, Lee H, Noh S, Bae BG, Shin JU, Park CO, et al. Retrospective Analysis on the effects of house dust mite specific immunotherapy for more than 3 years in atopic dermatitis. Yonsei Med J. 2016; 57:393–398.
48. Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006; 61:202–205.
49. Lee JH, Son SW, Cho SH. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol Res. 2016; 8:181–190.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download