Abstract
The immune system is continuously exposed to great amounts of different antigens from both food and intestinal microbes. Immune tolerance to these antigens is very important for intestinal and systemic immune homeostasis. Oral tolerance is a specific type of peripheral tolerance induced by exposure to antigen via the oral route. Investigations on the role of intestinal immune system in preventing hypersensitivity reactions to innocuous dietary and microbial antigens have been intensively performed during the last 2 decades. In this review article, we discuss how food allergens are recognized by the intestinal immune system and draw attention to the role of regulatory T (Treg) and B (Breg) cells in the establishment of oral tolerance and tolerogenic features of intestinal dendritic cells. We also emphasize the potential role of tonsils in oral tolerance induction because of their anatomical location, cellular composition, and possible usage to develop novel ways of specific immunotherapy for the treatment of allergic diseases.
Food allergy is described as “an adverse health effect that arises from a specific response that occurs reproducibly on exposure to a given food.” In contrast, food intolerance is a non-immune reaction that includes toxic, pharmacologic, metabolic, and undefined mechanisms.1 It is estimated that food allergy affects an average of 4% to 8% of children and 5% of adults worldwide.2 A detailed meta-analysis of 51 self-reported allergy studies to milk, egg, peanut, and seafood, revealed that the incidence of food allergy ranges from 3% to 35%. In another meta-analysis, which included 36-population-based studies of allergy to vegetables and fruits, 0.1% to 4.3% of individuals were allergic to fruits and tree nuts, less than 1% for wheat, soy, and sesame and from 0.1% to 1.4% for vegetables.3 Childhood allergies typically resolve by 3 years of age; however, only 11% of allergies to egg and 19% of allergies to milk resolve by the age of 4 years with about 80% of the above-mentioned food allergies resolving by the 16 years of age.4 Allergies to shellfish, fish, tree nuts, and peanut can be persistent, while childhood allergies to soy, wheat, egg, and milk usually resolve during childhood. It is worth to underline that the incidence of food allergies seems to be increasing over the last 2 decades. Data from the US Centers for Disease Control and Prevention concluded that prevalence of food allergies increased by 1.7% over the last 20 years. Surprisingly, there is no approved treatment for food allergies, other than allergen avoidance and emergency treatment in cases of accidental ingestion. As can be imagined, the patient's quality of life is affected because of anxiety and fear of unintentional ingestion.56 The need for new therapeutic approaches to eliminate or reduce the severity of allergic reactions upon ingestion is driving new studies that investigate the mechanisms of oral tolerance. One promising area of current investigation is the use of oral and sublingual allergen immunotherapy for treatment of food allergies.7
Understanding the mechanisms of allergic diseases may help unravel mechanisms that promote tolerance rather than desensitization in food allergy. Allergy is typically considered an inappropriate type 2-mediated immunological response, with increased numbers of allergen-specific T helper type 2 lymphocytes (Th2) and elevated allergen-specific IgE immunoglobulins.8 Th2 cells and innate type 2 lymphoid cells (ILC2) that express GATA Binding Protein 3 (GATA3) and produce interleukin-4 (IL-4), IL-5, IL-13 are involved in type 2 immunity. These cells promote basophil, mast cell, and eosinophil activation along with allergen-specific immunoglobulin E (IgE) production. Type 2 immunity has evolved to protect against parasite infections. However, when dysregulated, Th2 responses contribute to the development of allergic diseases that usually require 2 phases. In the first phase, allergen presentation by dendritic cells to naïve T cells results in the generation of Th2 cells. IL-4, IL-5, and IL-13 produced by newly differentiated Th2 cells induce immunoglobulin heavy chain class switching to IgE. Later, IgE produced by plasma cells binds to high-affinity IgE receptors on mast cells and basophils. Subsequently, when exposure to the allergen occurs for the second time, the allergen binds to and crosslinks IgE antibodies present on the surface of basophils and mast cells. Activated cells release a plethora of inflammatory mediators, such as histamine and leukotrienes, which contribute to symptoms associated with allergic inflammation. In the skin, symptoms manifest as swelling, erythema, and pruritus, while reduced lung function, airway hypersensitivity and remodeling are associated with respiratory allergies.8
Besides Th2 cells, newly described CD4+ T-helper subsets, including Th9, Th17, and Th22, may play a role in allergic diseases. Initially, IL-9 was described as a Th2 cell-derived cytokine. Later, a separate Th9 subset of cells was defined. Th9 cells differentiate in the presence of transforming growth factor beta (TGF-β) and IL-4. In the ovalbumin (OVA)-induced model of airway inflammation, IL-9 neutralization strongly reduces allergic symptoms,9 and the transfer of OVA-specific Th9 cells into T-cell deficient mice promotes asthma symptoms. In humans, increased numbers of Th9 cells have been shown in the blood of allergic patients.10 In summary, protective and inflammatory roles of Th9 cells in mouse models and humans have been described.
As soon as naïve T cells are activated by IL-6, TGF-β, and IL-23, their differentiation is polarized into Th17 cells. Polarizing cytokines activate the transcription factor STAT3, which further induces the RAR-related orphan steroid receptor (RORγt), a key transcriptional regulator of Th17 cells. Th17 cells produce the main cytokines IL-17A and IL-17F. Furthermore, Th17 cells may produce IL-22 that is responsible for epithelial cell homeostasis and antimicrobial defense, along with tumor necrosis factor alpha (TNFα) and granulocyte-monocyte colony-stimulating factor (GM-CSF) that recruit and activate neutrophils.11 Expansion of Th17 cells is supported by IL-23, and Th17 cells are notably abundant in gut mucosal tissues. The main function of Th17 cells is the induction and coordination of neutrophil responses to ingest and kill extracellular microbes. However, psoriasis, inflammatory bowel disease, rheumatoid arthritis, and multiple sclerosis are associated with inappropriate Th17 cell responses.11
Another subset of T-helper cells that are closely related to Th17 cells is Th22 cells. The main cytokine produced by Th22 cells is IL-22. Because of the preferential expression of IL-22 receptor 1 (IL-22R1) on cells of epithelial origin, IL-22 is a unique cytokine produced by immune-cells and acts only on tissue cells.12 The first immune mediated disorder that has been associated with IL-22 is psoriasis. IL-22 expression is up-regulated in the lesional skin as compared to the non-lesional psoriatic and healthy skin.12 The anti-psoriatic therapy significantly decreases IL-22 expression in the lesional skin. Moreover, IL-22 levels are also elevated in the inflamed skin of atopic dermatitis (AD) patients.12 In the gastrointestinal track, the role of IL-22 has been mainly investigated in inflammatory bowel disease, in which IL-22 is considered to play a protective role by acting on intestinal epithelial cells and inducing the production of antimicrobial peptides that modulate the colonic microbiota, expression of mucus-associated molecules, and reconstitution of the mucus-producing goblet cells, as well as epithelial cell proliferation that contributes to the regeneration and repair of epithelial layers.13 Interestingly, increased levels of IL-22 mRNA and protein have been reported in the blood of asthmatic patients and in murine asthma models.14 IL-22 has a dual role in asthma-associated lung inflammation. Depending on the route and time of administration, IL-22 may reduce or exacerbate lung pathology and inflammation. Taking into account that Th22 cells seem to play a role in allergic diseases, it would be interesting to investigate the role of Th22 cells in food allergy.
Another cell type that influences responses during allergic inflammation is epithelial cells. Intestinal epithelial cells form a tight and selective barrier that allow highly controlled, paracellular, and transcellular transport of molecules or antigens, and are necessary for the induction of appropriate immune responses in the gut. Additionally, it has been described that via production of thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, epithelial cells may enhance type 2 immune responses. For example, TSLP not only modifies dendritic cells (DC) to preferably induce Th2-antigen-specific lymphocytes but also enhances basophil hematopoiesis. Epithelial cell secretion of IL-25 enhances the differentiation of mast cells and basophils, and is associated with allergen sensitization in humans with asthma.15 Finally, epithelial-derived IL-33 has been shown to be essential for peanut sensitization in the mouse and may increase permeability of mucosal tissues.16 In addition to the effects on Th2 cells, TSLP, IL-25, and IL-33 induce activation and IL-4, IL-13, and IL-5 production from the recently discovered type 2 ILCs.17
Induction of tolerance to food allergens takes place in the gut and gut-associated lymphoid tissue (GALT), where the highest fraction of immune system cells inside the body resides. Mesenteric lymph nodes (MLN) and Peyer's patches (PP) are the main components of the organized GALT. Payer's patches are lymphoid-cell accumulation areas found in the submucosa, primarily the small intestine, and consist of B-cell follicles and surrounding T-cell areas. Immediately above the B-cell follicles and below the follicle-associated epithelium, a region known as the subepithelial dome is present. This dome is covered by single layer of columnar-follicle-associated epithelium that separates it from the intestinal lumen. These epithelial cells produce lower levels of digestive enzymes, possess less pronounced brush borders, and are called “microfold (M) cells.” The second compartment, MLN, are largest in the body and drain the intestine via lymphatics.18
Below the single epithelial cell layer are multiple types of immune cells, including CD4+T and CD8+T effector and regulatory cells, B cells, γδ T cells, phagocytes, and antigen presenting cells that recognize and clear pathogens, thus contributing to effector mechanisms. Immune responses in the gut are efficiently induced in PP and MLN. For the induction of oral tolerance, PP have been shown to be dispensable, while MLN are required.19
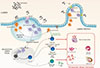
The initial contact between immune cells and antigens from the lumen is a critical step in the induction of intestinal immune responses. Food proteins are processed into small peptides and amino acids that in later stages will be absorbed by intestinal epithelial cells. This process starts in the stomach and continues through the duodenum and jejunum. However, immunologically active proteins may escape the process of digestion. In the intestine, they are 3 types of cells specialized in antigen uptake: M cells, intestinal epithelial cells, and dendritic cells (Figure). It has been recognized that M cells are only cells that transport antigens that come into contact with intestinal immune cells. Interestingly, M cells do not express major histocompatibility complex (MHC) class II molecules or present antigens. Their role is strictly restricted to antigen transport to antigen-presenting DC that reside below the epithelial cell layer.18 Intestinal epithelial cells can express MHC class I and II molecules, while they produce and respond to various cytokines. In contrast to antigen-presenting cells, enterocytes do not express co-stimulatory molecules that provide a second signal for full T-cell activation and therefore may be good candidates as tolerogenic antigen-presenting cells that contribute to the development of oral tolerance.18 In addition, soluble, low molecular weight antigens can be transported from the lumen to tolerogenic DC.20 However, well-known food allergenic molecules, such as lipid transfer proteins (LTP), may be transported intact by epithelial cells.21
Prevention and treatment of allergy, asthma, and other non-allergic diseases, such as autoimmunity and organ transplantation, where the immune system is dysregulated may be possible if immunological tolerance can be induced.22 In general, immunological tolerance can develop to any substance that otherwise may activate the immune system. Mechanisms of immunological tolerance are complex and influenced by the following factors: antigen dose, structure, time and route of exposure; genetic susceptibility, composition, and metabolic activity of the microbiome. Administration of increasing doses of specific antigen in the form of allergen-specific immunotherapy (AIT) may provide long-term curative treatment. Two main types of AIT, SCIT (subcutaneous immunotherapy) and SLIT (sublingual immunotherapy), exist when antigens are applied subcutaneously and sublingually, respectively. In cases of aeroallergens and venom allergens, it has been shown that SCIT and SLIT induce allergen-specific Treg cells, which are critical to the success of AIT.23 Supporting data are accumulating for food allergy and other immunotherapy routes, including oral immunotherapy (OIT), which will be discussed in the next section. The ultimate goal of immunotherapy is the induction of clinical tolerance defined as the long-term, established absence of allergic symptoms after allergen exposure. On the other hand, immunological tolerance requires suppression of allergen-specific B and T cell effector responses, changes in humoral responses including development of IgG4 antibodies, changes in the threshold dose required for activation of basophils and mast cells and switching from Th2 to Th1 response with accompanying induction of specific-Treg cells.
In food allergy, tolerance is classically described as the absence of allergic symptoms upon food intake after a period of food avoidance. However, in most cases, only patients remaining on maintenance doses do not develop reactions and food desensitization rather than tolerance is accomplished. Despite a growing literature in the field of food allergy, it is not yet clear why the majority of individuals are immunologically tolerant to food antigens, while few individuals develop allergic sensitization. In the present review, we focus on the mechanisms of oral tolerance and the role of regulatory cells in this process. In 1911, Wells and Osborne for the first time described a phenomenon, termed “oral tolerance,” by showing that guinea pigs could not develop anaphylaxis to proteins of corn or oats that were present as components of their diet.24 Oral tolerance is a mechanism by which potentially antigenic substances that are administered orally do not elicit cellular or humoral immune responses.25 In mouse models of food allergy, mice fed with a single high antigen dose (50-100 mg) or with 5 times daily feeds of low antigen dose (0.5-1 mg) can become non-responsive to administered antigen. Low doses favor the induction of Treg cells, while higher doses support the anergy and deletion of antigen-specific T cells.26 Interestingly, in the Learning Early About Peanut Allergy study it has been shown that early oral introduction of peanuts could prevent allergy non-sensitized as well as sensitized infants.27
To elicit mucosal immune responses, antigen (Ag) has to be sampled and presented by antigen-presenting cells. Antigens may be acquired via several different mechanisms. First of all, M cells that overlie PP and goblet cells may contribute to selective sampling of viral and bacterial antigens. On the other hand, soluble antigens are mainly transported across epithelial cells by endocytosis and subsequently transported via immune cells to mesenteric lymph nodes.28 DC, which are professional antigen-presenting cells that orchestrate immune responses by linking innate and adaptive immunity, may contribute to induction of peripheral tolerance. Early experiments suggested that only immature DC contribute to generation of Treg cells and mature DC provide stimulation for induction of several different T-helper effector cell subsets. Nowadays, the current knowledge suggests that mature DC are also capable of inducing T reg cells via different mechanisms, including cytokines (TGF-β, IL-10), enzymes (IDO, idoleamine 2,3-dioxygenase; RALHD, retinal dehydrogenase) and metabolites (RA, retinoic acid).29
In the gut, DC that express CD11b and CX3CR1 can extend dendritic processes between epithelial cells to sample antigen on the apical surface of epithelial cells.30 These DC, which are monocyte-derived cells, do not express CCR7 constitutively, do not migrate to MLN, and are transcriptionally more related to macrophages than to DC.31 However, CD11b+CX3CR1+cells express high levels of IL-10.32 Additionally, 2 additional tolerance-associated DC have been described within the lamina propria: CD11c+CD103+CX3CR1- and CD11c+CD103-CX3CR1+cells.33 In contrast to CD11b+CX3CR1+DC, CD11c+CD103+CX3CR1- DC constitutively express CCR7 and migrate to mesenteric lymph nodes under homeostatic conditions. Moreover, CD11c+CD103+CX3CR1- DC do not sample antigens by extending dendrites between tight junctions of epithelial cells, they rather sample antigens that were transported by epithelial cells, goblet cells, or M cells.203233 In addition to these features, CD103+CX3CR1- DC express high levels of TGF-β and RALDH that is involved in the production of RA from vitamin A. CD103+CX3CR1- DC can also express IDO and both of these metabolic activities contribute to active promotion and development of Treg cells from naïve T cells in mesenteric lymph nodes.34 Importantly, Treg cells differentiated with the help of CD103+CX3CR1- DC up-regulate, in a RA-dependent manner, α4β7 integrin, and CCR9 receptor that are responsible for T-cell homing from MLN to the lamina propria. Furthermore, CD103+CX3CR1- DC not only influence T-cell responses but also direct gut-homing-IgA-secreting plasma cells.35 The fact that different subsets of DC's are present in the lamina propria may suggest that they play distinct roles in the induction of oral tolerance. It is possible that CD103+DC migrate to MLN where they contribute to the development of gut-homing Treg cells. Once Treg cells are primed in MLN and move back to lamina propria, they interact with CX3CR1+DC that lack migratory capacity and can be expanded locally.36
Treg cells are potent immune regulating cells that play a central role in controling immune responses to allergens. The main mechanisms underpinning Treg cell effects include production of inhibitory cytokines (IL-10, TGF-β and IL-35), effector cells cytolysis (via secretion of granzymes A and B), direct targeting of DC via inhibitory PD-1 (programmed cell dead protein 1) and CTLA4 (cytotoxic T-lymphocyte-associated protein 4) cell surface molecules and metabolic disruption of effector cells (CD25, cAMP, adenosine, CD39, and CD73).37 Interestingly, Th3 cells may promote the development of induced Treg cells (iTregs) via TGF-β production.38 IL-10 is a key cytokine that controls immune response. IL-10 directly inhibits IFN-γ and IL-2 secretion by Th1 cells and IL-4/IL-5 production by Th2 cells. Prolonged immune responses against antigens, which in several cases lead to tissue damage and inflammation, were observed in IL-10-deficient mice.39
Administration of antigens by the oral route induces 2 different populations of Treg cells: CD4+CD25+ forkhead box P3 (Foxp3)+ Treg cells and Th3 cells. The main mechanism of suppression provided by Th3 cells is dependent on the inhibitory cytokine TGF-β as Th3 cells do not express Foxp3+ or CD25 but do express LAP (latency associated peptide) that forms a complex with TGF-β. In contrast, CD4+CD25+Foxp3+ T cells, called iTregs, are involved in the inhibition of allergen specific Th2 cells,40 promotion of allergen-specific IgG4 production rather than allergen-specific IgE41 and generation of tolerogenic DC.
Despite the fact that specific mechanisms for oral tolerance maintenance by T reg cells within the lamina propria remain to be clarified, studies with specific deletion of Foxp3+-induced Treg cells proved that they are mandatory for oral tolerance.36 Humans with mutations in the Foxp3 locus immune dysregulation, polyendocrinopahty, enteropathy, X-linked [IPEX] syndrome)42 and Foxp3-mutant mice display failures in oral tolerance, which leads to inappropriate responses to many types of antigens, including food antigens. Moreover, iTreg cells are essential, while thymic-derived natural Treg cells which also express Foxp3 are dispensable for oral tolerance as shown by deletion studies in mice.43 This hypothesis is additionally supported by the fact that iTreg cells rather than nTreg cells are involved in the control of Th2-related mucosal inflammation.44 Results described above suggest that Treg cells play a role in the constitutive suppression of responses to antigens encountered in GALT. Interestingly, studies in children who outgrew milk allergy revealed reduced proliferation of milk-specific T effector cells and increased frequency of CD4+CD25+ Treg cells following allergen challenge, suggesting that not only maintenance but also the development of oral tolerance requires Treg cells.45
Oral tolerance can also be influenced by CD8+T cells and these cells are reduced in the lamina propria of inflammatory bowel disease patients compared to healthy controls.46 However, these cells do not seem to provide tolerance against allergic inflammation driven by Th2 cells and are not necessary for the development of oral tolerance.47
Similarly to Treg cells, Breg cells suppress effector T cells and other lymphocytes via production of IL-10, IL-35, and TGF-β and involved in supporting immunological tolerance.48 Mouse models, where animals lack IL-10-producing B cells, proved that deficiency in B cell regulatory function results in chronic inflammation. However, the role of Breg cells in oral tolerance is not yet well established. Our group has shown that IL-10-secreting Breg (Br1) cells contribute to peripheral allergen tolerance. In bee venom allergic patients receiving AIT, the frequency of phosphilipase A2-specific, IL-10-producing B cells increases. Additionally, IgG4, a non-inflammatory immunoglobulin isotype that blocks IgE-mediated mast cells and basophils degranulation, was specifically produced by Br1 cells.49 The potential mechanism through which IL-10-producing B cells may induce tolerance was investigated using IL-10-overexpressing B cells. These B cells could suppress DC maturation and T-effector cell proliferation, and secret less IgE. Furthermore, IL-10+B cells produced the anti-inflammatory IL-1 receptor antagonist and vascular endothelial growth factor, with reduced production of pro-inflammatory cytokines.50 A recent study has suggested that mesenteric IL-10-producing CD5+ Breg cells may play a role in the regulation of IgE-mediated anaphylaxis following challenge with cow's milk allergens.51 In patients with non-IgE-mediated food allergy and AD, IL-10 producing B reg cells do not proliferate upon antigen stimulation, while these cells proliferate when obtained from tolerant volunteers. Similar responses have been shown for TGF-β producing B regulatory type 3 cells, suggesting that Breg cells may contribute to the maintaince of tolerance in both IgE-mediated and non-IgE-mediated food allergies.
A significant increase in the levels of IgG4 specific for food antigens have been observed in many oral immunotherapy (OIT) studies. Despite an initial increase in food-specific IgE levels, the IgE levels usually decrease by the end of OIT and SLIT studies.5253 An increase in IgG4 and a decrease in IgE might be due to the down-regulation of IL-4 production and up-regulation of IL-10 production because IL-10 induces heavy chain immunoglobulin isotype switching to IgG4 and because IL-4 induces IgE. A possible role of food allergen-specific IgG4 in maintaining desensitization and supporting tolerance was suggested in studies where peanut-specific IgG4 levels decreased after discontinuing maintenance dosing with allergen.54 As in OIT and SLIT, IgG4 levels in patients undergoing SCIT can be increased 10-100 times. However, clinical improvement does not always correlate with IgG4 concentration in serum.
Additionally, mucosal IgA produced by B cells prevents uptake of antigen across the epithelium and may protect against immunogenicity to food antigens.55
Different cells that play a role in the induction and maintenance of oral tolerance might be influenced by the presence of gut microbiota.56 It is well known that gut microbiota shape the intestinal immune system and impact barrier function.57 Mice that are bred under germ-free or sterile conditions do not fully develop tolerance mechanisms and similarly to mice treated with antibiotics or mice lacking Toll-like receptors (TLRs), and are characterized by increased susceptibility to sensitization against food allergens.58 In addition, it has been shown that in the context of allergic inflammation, IgE production and basophil development are suppressed in the presence of signals provided by commensal microbes.59 Furthermore, certain bacterial strains, such as Bifidobacterium longum 35624, Clostridia and Bacterioides fragilis can induce intestinal Treg cells that are capable of suppressing food allergy and colitis.6061 Pattern-recognition receptor activation on DC seems to be an important mechanism by which intestinal microbes may promote Treg cell differentiation.62 On the other hand, intestinal microbes, such as segmented filamentous bacteria, can also promote the development of Th17 rather than Treg cells.
In murine experimental models of food allergy, it has been shown that mouse strains more susceptible to the development of food allergy possess a microbiome that differs from wild-type animals or non-susceptible but allergen-sensitized mice.5863 Interestingly, more severe responses to food allergen challenge are observed in gnotobiotic mice reconstituted with microbes from allergic animals.64 In contrast, when gnotobiotic mice are colonized with microbiota from non-allergic children, these mice can be protected against allergen sensitization to milk allergens.65 These results suggest that the microbiota may play both protective and detrimental roles in the development of food allergy.
One of the possible mechanisms to explain this phenomenon is that the microbiota normally instructs Treg cells to suppress Th2-derived responses. In the absence of these instructions, Treg cells change their phenotype to one which does not suppress food allergy but can actually participate in its development.66 Support for this hypothesis comes from experiments where Treg cells from food allergy in susceptible, but not from resistant mice, had a mixed Treg-Th2 phenotype as they expressed the transcription factor GATA-3 and produced the cytokine IL-4. In addition, deletion of Th2-cytokine genes specifically from Foxp3+ T cells prevents the development of food allergy.66 Finally, another study has demonstrated that in the absence of an intestinal microbiota, Treg cells do not express RORγt, which favors the accumulation of GATA3+ Treg cells as well as Th2 cells.67
In addition to direct contact with microbial structures, the intestinal microbiome is metabolically active, and microbial metabolites have been shown to exert significant effects on host immune signaling networks.68 Short-chain fatty acids (SCFA) are produced via microbiome fermentation of dietary fibers, and SCFA can promote DC cell regulatory activity, resulting in induction of Treg cells and IL-10-secreting T cells.69 In addition to SCFA, the microbiome secretes a wide range of other biologically important metabolites. For example, histamine is secreted by gut microbes, and mucosal histamine levels are increased in patients with irritable bowel syndrome and inflammatory bowel disease.70 Histamine modifies chemokine and cytokine secretion by DC via the G protein-coupled receptor histamine receptor 2, while microbes secreting histamine exert immunoregulatory effects in mouse models.71 The interactions between microbiome and dietary factors, which influence oral tolerance, is currently an exciting area of study, particularly in food allergy.
The palatine tonsils (PTs) are part of the mucosa-associated lymphoid tissue (MALT) in the human pharynx, which is called “Waldeyer's ring.” Besides palatine tonsils, Waldeyer's ring consists of the nasopharyngeal tonsil (adenoid attached to the roof of the pharynx), paired tubal tonsils (at the openings of the Eustachian tubes), and lingual tonsil (at the back of the tongue). PTs are easily accessible and the best studied among all the tonsils. PTs are positioned at the entry site of both the respiratory and gastrointestinal tracks, where foreign antigens and substances from food and air come into contact with the mucosal tissues, just before they are exposed to digestive enzymes and acidic gastric secretions. This is the strategic region where the immune response to diverse antigens that enter the body through the mouth and nose are initiated. Adenoids as well as PT can be removed; however, fully functional lingual and pharangeal tonsils probably take over their role. The characteristic feature of PT is the formation of deep tubular crypts that extend the external surface of the tonsil up to 300 cm2. Long-term contact with antigens and direct stimulation of immune cells with food and air-borne allergens are possible due to this highly cryptic surface. The outer surface of the PT is covered with stratified squamous non-keratinized epithelium. However, the crypt epithelium is called “lymphoepithelium” as it is infiltrated with many of the non-epithelial cells, mainly lymphocytes. In the reticulated epithelium, cells which functionally match intestinal M-cells are found. As they possess remarkable potential to transcytose a broad range of particles and soluble material without their degradation, they translocate antigens to subepithelial lymphoid tissue. Immune cells, mainly lymphocytes, are found in all compartments of the tonsils, including the lymphoepithelium, the inter-follicular regions, and the follicles. Among the intra-epithelial lymphocytes, 50% are B cells with a smaller number of T cells, mostly CD4+ rather than CD8+ T cells. Similarly, secondary lymphoid follicles are mainly populated by B cells, where they undergo intense maturation and dynamic differentiation. In addition to B cells, follicular T cells and follicular DC are found in the germinal center. Interestingly, McClory et al.72 identified 5 tonsillar T cell developmental intermediates and proved that each of them resembles its thymic counterpart. With this work, it has been suggested that a full spectrum of T cell development stages may take place in human tonsils.72 Tonsils have also been shown to be the potential first-line organ involved in tolerance via the generation of allergen specific Foxp3+ T regulatory cells. It has been shown that there is a higher number of Bet v 1-specific CD4+Foxp3+ Treg cells in PT as compared to peripheral blood.73 Not only does tolerance induction occur in tonsils, but also allergen-specific T cell tolerance can be disrupted in the tonsil upon stimulation with TLR4, TLR8, and pro-inflammatory cytokines.74 From an immunological point of view, PT are a very interesting organ, but not yet fully investigated. It may be possible to develop novel immunotherapy protocols that target these organs as a potential treatment of food allergy.
Dynamic interactions between host immune cells, microbiome, dietary factors, and food allergens determine whether allergy or tolerance develops. Despite a growing knowledge about food allergies, immune responses to food allergens diverge from default Treg cell-mediated suppressive responses to Th2-mediated, IgE-induced responses, still needs to be investigated. Oral tolerance is an active regulatory immune response, and mechanisms inducing oral tolerance are summarized here that it is mediated by allergen-specific Treg cells generated by mucosal DC. Intestinal mucins and cytokines coming from epithelial cells and innate lymphoid cells contribute to tolerance by modifying the phenotype of gastrointestinal DC. In addition, mucosal epithelial barrier regulation may play an important role.75 Oral tolerance by early dietary introduction can prevent peanut allergy in infants at high risk of peanut allergy. As for humoral mechanisms, generation of allergen-specific IgG4 is especially associated with the development of tolerance to foods in humans. Clinical tolerance induced by immunotherapy is associated with changes in basophils, IgG4, allergen-specific Th2 cells, and allergen-specific cells with regulatory markers.7677 A more complete understanding of mechanisms underlying the induction of oral tolerance with immunotherapy or natural tolerance to food allergens in healthy individuals will help develop better treatment options for patients suffering from food allergies.
Figures and Tables
Figure
Role of regulatory cells in the induction of oral tolerance. Food antigens are acquired by the immune system via several different mechanisms. First of all, dendritic cells (DC) expressing CD11b and CX3CR1 extend dendritic processes between epithelial cells and sample antigens from lumen (1). As CX3CR1+CD11b+ DC do not express CCR7 or migrate to mesenteric lymph node, they mainly contribute to local, antigen-specific expansion of regulatory cells. Additionally, M cells localized in Payer's patches (2), intestinal cells via paracellular (3), or transcellular route (4), and goblet cells (5) might contribute to antigen sampling. Antigens are uptaken by CD11C+CD103+DC. DC expressing CD11c and CD103 co-express CCR7 and migrate to MLN under homeostatic conditions. In MLN produced by of TGF-beta, IL-10, RALDH, and IDO contribute to active promotion and development of Treg and Breg cells. Treg cells differentiated in MLN by CD11C+CD103+ DC up-regulate α4β7 integrin and CCR9 receptor that are responsible for T-cell homing from MLN to the lamina propria. In lamina propria, CD25-Foxp3--, IL-10-and TGF-β-producing Th3/Tr1 cells, CD25+Foxp3+ α4β7+CCR9+ iTreg cells, and IL-10+IgG4+ Breg cells suppress Th2-dependent allergic inflammation by decreasing mast cell activation, IL-4/IL-5/IL-13 production from ILC2 and Th2 cells, and IgE production from plasma cells.
ACKNOWLEDGMENTS
The authors' laboratories are supported by the Swiss National Science Foundation No. 320030-159870, European Commission's Seventh Framework Programme under grant agreement No. 261357 (MeDALL), European Commission's, Seventh Framework programme under grant agreement No. 260895 (PREDICTA) and Christine Kühne-Center for Allergy Research and Education (CK-CARE).
References
1. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014; 69:1008–1025.
2. Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014; 133:291–307.
3. Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C, et al. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008; 121:1210–1218.e4.
4. Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007; 120:1413–1417.
5. Ahn K. The usefulness of component-resolved diagnostics in food allergy. Allergy Asthma Immunol Res. 2014; 6:103–104.
6. Song TW. Diagnostic Decision Points of Specific IgE Titers in Patients With Food Allergy: Are They Appropriate in All Clinical Settings? Allergy Asthma Immunol Res. 2015; 07. 7:309–311.
7. Cavkaytar O, Akdis CA, Akdis M. Modulation of immune responses by immunotherapy in allergic diseases. Curr Opin Pharmacol. 2014; 17:30–37.
8. Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012; 18:736–749.
9. Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010; 11:527–534.
10. Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012; 129:1000–1010.e3.
11. Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Defining the human T helper 17 cell phenotype. Trends Immunol. 2012; 33:505–512.
12. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004; 21:241–254.
13. Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008; 118:534–544.
14. Farfariello V, Amantini C, Nabissi M, Morelli MB, Aperio C, Caprodossi S, et al. IL-22 mRNA in peripheral blood mononuclear cells from allergic rhinitic and asthmatic pediatric patients. Pediatr Allergy Immunol. 2011; 22:419–423.
15. Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013; 131:187–200.e1-8.
16. Turnquist HR, Thomson AW. IL-33 broadens its repertoire to affect DC. Eur J Immunol. 2009; 39:3292–3295.
17. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015; 135:626–635.
18. Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003; 3:331–341.
19. Spahn TW, Weiner HL, Rennert PD, Lügering N, Fontana A, Domschke W, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002; 32:1109–1113.
20. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012; 483:345–349.
21. Tordesillas L, Gómez-Casado C, Garrido-Arandia M, Murua-García A, Palacín A, Varela J, et al. Transport of Pru p 3 across gastrointestinal epithelium - an essential step towards the induction of food allergy? Clin Exp Allergy. 2013; 43:1374–1383.
22. Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest. 2014; 124:4678–4680.
23. Jutel M, Kosowska A, Smolinska S. Allergen Immunotherapy: Past, Present, and Future. Allergy Asthma Immunol Res. 2016; 8:191–197.
24. Wells HG, Osborne TB. The biological reactions of the vegetable proteins. J Infect Dis. 1911; 8:66–124.
25. Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005; 115:3–12.
26. Chen YH, Weiner HL. Dose-dependent activation and deletion of antigen-specific T cells following oral tolerance. Ann N Y Acad Sci. 1996; 778:111–121.
27. Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015; 372:803–813.
28. Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy, and immunotherapy: implications for future treatment. J Allergy Clin Immunol. 2008; 121:1344–1350.
29. Schiavi E, Smolinska S, O'Mahony L. Intestinal dendritic cells. Curr Opin Gastroenterol. 2015; 31:98–103.
30. Berin MC, Shreffler WG. Mechanisms underlying induction of tolerance to foods. Immunol Allergy Clin North Am. 2016; 36:87–102.
31. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009; 206:3101–3114.
32. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007; 8:1086–1094.
33. Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009; 31:513–525.
34. Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010; 59:595–604.
35. Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006; 314:1157–1160.
36. Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011; 34:237–246.
37. Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013; 14:307–308.
38. Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007; 178:179–185.
39. Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013; 4:129.
40. Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010; 40:1232–1240.
41. Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008; 63:1455–1463.
42. Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007; 132:1705–1717.
43. Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008; 29:114–126.
44. Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012; 482:395–399.
45. Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009; 123:43–52.e7.
46. Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005; 174:5814–5822.
47. Arnaboldi PM, Roth-Walter F, Mayer L. Suppression of Th1 and Th17, but not Th2, responses in a CD8(+) T cell-mediated model of oral tolerance. Mucosal Immunol. 2009; 2:427–438.
48. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015; 42:607–612.
49. van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013; 131:1204–1212.
50. Stanic B, van de Veen W, Wirz OF, Rückert B, Morita H, Söllner S, et al. IL-10-overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol. 2015; 135:771–780.e8.
51. Kim AR, Kim HS, Kim DK, Nam ST, Kim HW, Park YH, et al. Mesenteric IL-10-producing CD5(+) regulatory B cells suppress cow's milk casein-induced allergic responses in mice. Sci Rep. 2016; 6:19685.
52. Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011; 127:640–646.e1.
53. Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009; 124:292–300. 300.e1–300.e97.
54. Rachid R, Umetsu DT. Immunological mechanisms for desensitization and tolerance in food allergy. Semin Immunopathol. 2012; 34:689–702.
55. Karlsson MR, Johansen FE, Kahu H, Macpherson A, Brandtzaeg P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy. 2010; 65:561–570.
56. Kim BJ, Lee SY, Kim HB, Lee E, Hong SJ. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol Res. 2014; 6:389–400.
57. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012; 336:1268–1273.
58. Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004; 172:6978–6987.
59. Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012; 18:538–546.
60. Lyons A, O'Mahony D, O'Brien F, MacSharry J, Sheil B, Ceddia M, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy. 2010; 40:811–819.
61. Konieczna P, Ferstl R, Ziegler M, Frei R, Nehrbass D, Lauener RP, et al. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS One. 2013; 8:e62617.
62. Konieczna P, Schiavi E, Ziegler M, Groeger D, Healy S, Grant R, et al. Human dendritic cell DC-SIGN and TLR-2 mediate complementary immune regulatory activities in response to Lactobacillus rhamnosus JB-1. PLoS One. 2015; 10:e0120261.
63. Rodriguez B, Prioult G, Bibiloni R, Nicolis I, Mercenier A, Butel MJ, et al. Germ-free status and altered caecal subdominant microbiota are associated with a high susceptibility to cow's milk allergy in mice. FEMS Microbiol Ecol. 2011; 76:133–144.
64. Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013; 131:201–212.
65. Rodriguez B, Prioult G, Hacini-Rachinel F, Moine D, Bruttin A, Ngom-Bru C, et al. Infant gut microbiota is protective against cow's milk allergy in mice despite immature ileal T-cell response. FEMS Microbiol Ecol. 2012; 79:192–202.
66. Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015; 42:512–523.
67. Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015; 349:989–993.
68. Frei R, Lauener RP, Crameri R, O'Mahony L. Microbiota and dietary interactions: an update to the hygiene hypothesis? Allergy. 2012; 67:451–461.
69. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014; 121:91–119.
70. Smolinska S, Jutel M, Crameri R, O'Mahony L. Histamine and gut mucosal immune regulation. Allergy. 2014; 69:273–281.
71. Frei R, Ferstl R, Konieczna P, Ziegler M, Simon T, Rugeles TM, et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J Allergy Clin Immunol. 2013; 132:194–204.
72. McClory S, Hughes T, Freud AG, Briercheck EL, Martin C, Trimboli AJ, et al. Evidence for a stepwise program of extrathymic T cell development within the human tonsil. J Clin Invest. 2012; 122:1403–1415.
73. Palomares O, Rückert B, Jartti T, Kücüksezer UC, Puhakka T, Gomez E, et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol. 2012; 129:510–520.e1-9.
74. Kücüksezer UC, Palomares O, Rückert B, Jartti T, Puhakka T, Nandy A, et al. Triggering of specific Toll-like receptors and proinflammatory cytokines breaks allergen-specific T-cell tolerance in human tonsils and peripheral blood. J Allergy Clin Immunol. 2013; 131:875–885.
75. Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Rückert B, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2016.
76. Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016.
77. Wawrzyniak P, Akdis CA, Finkelman FD, Rothenberg ME. Advances and highlights in mechanisms of allergic disease in 2015. J Allergy Clin Immunol. 2016; 137:1681–1696.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download