Abstract
Purpose
Chronic rhinosinusitis with nasal polyps is a chronic inflammatory disease with markedly increased eosinophils, Th2-type lymphocytes, fibroblasts, and goblet cells. Fungi are commonly associated with airway inflammatory diseases, and thymic stromal lymphopoietin (TSLP) is important in the development of Th2 inflammatory responses. The aim of this study was to investigate the interaction between airborne fungi and nasal fibroblasts in TSLP mRNA and protein expression.
Methods
Inferior turbinate and nasal polyp fibroblasts were stimulated with Alternaria and Aspergillus, respectively, for 48 hours, and TSLP mRNA and protein expressions were measured. The reverse transcriptase polymerase chain reaction was performed for the Toll-like receptor (TLR) mRNA expression of the nasal fibroblasts. To determine the role of TLR in the induction of TSLP, the fibroblasts were transfected with siRNA against TLR2 and TLR5.
Results
Alternaria induced TSLP mRNA and protein expression in both inferior turbinate and nasal polyp fibroblasts. The nasal polyp fibroblasts responded more strongly to the fungi. TLR2 and TLR5 mRNA expressions were significantly increased with fungal stimulation and TSLP production was significantly inhibited by siRNA against TLR2.
Conclusions
The results of this study show that TSLP expression could be induced in nasal fibroblasts by exposure to Alternaria and that TLR2 may be involved in the process. The promotion of TSLP production in nasal fibroblasts by airborne fungi may facilitate the development or exacerbation of Th2-type nasal inflammation, especially in CRS with nasal polyps.
Thymic stromal lymphopoietin (TSLP) is an IL-7-line cytokine produced mainly by epithelial cells, epidermal keratinocytes, smooth muscle cells, fibroblasts, and dendritic cells.12 TSLP is an important factor linking responses at interfaces between the body and the environment to the Th2 response.2 It stimulates myeloid dendritic cells, leading to the differentiation of naïve T cells toward the Th2 cells that produce IL-4, IL-5, and IL-13 as well as TNF-α. Therefore, TSLP plays a key role in the development of allergic inflammation.3 However, little is currently known regarding factors that control the production of TSLP.
TSLP expression is increased in allergic rhinitis and nasal polyps, and the TSLP expression level is significantly greater in nasal polys, irrespective of whether nasal polyps are atopic or non-atopic, than in the allergic rhinitis.45 The immunoreactivity for TSLP has been detected in nasal epithelial cells, endothelial cells, fibroblasts, and inflammatory cells in nasal polyps.5 Fibroblasts are major structural components of nasal mucosa, which confer mechanical strength by providing a supporting framework of extracellular matrix and play an important role as a source of chemical mediators in the initiation and amplification of inflammatory reaction.6 Nasal polyp fibroblasts produce TSLP in response to stimulation by TNF-α and IL-1β via distinct signal transduction pathways, including NF-κB.7
Fungal spores are continuously inhaled and deposit in normal and patient's nasal mucosa without harmful effects. Some pathogenic fungi have been associated with airway inflammatory diseases, such as bronchial asthma and rhinosinusitis. Their enzymatic activity leads to the production of inflammatory chemical mediators through the interaction with Toll-like receptors (TLRs) and induces the production of TSLP through protease-activated receptor-2 (PAR-2).89 However, the interaction between airborne fungi and nasal fibroblasts has not been extensively researched. In this study, we investigated whether airborne fungi activate nasal fibroblasts to produce TSLP and the role of TLRs in the production of TSLP.
Primary nasal fibroblasts were isolated from 8 chronic rhinosinusitis with nasal polyp patients which removed during endoscopic sinus surgery, and from inferior turbinates of 5 septal deviation patients taken during septal surgery. The diagnosis of chronic rhinosinusitis (CRS) with nasal polyps was made according to the European Position Paper on rhinosinusitis and nasal polyps.10 The subjects were excluded if they had an allergy or asthma, had received systemic or topical steroids, or had taken antibiotics or other medications during the 4 weeks preceding the study. Allergy status was defined using the skin prick test or multiple allergen simultaneous test-chemiluminescent assay (Green Cross Corp., Yongin, Korea). The study was approved by the Institutional Review Board of Daegu Catholic University Medical Center. Each subject signed a consent form that outlined the objectives of the research and experiments.
Specimens were aseptically collected and cut into 0.5 mm fragments and cultured in Dulbeco's modified Eagle's medium F-12 (DMEM/F-12) (Gibco, Grand Island, NY, USA) that contained 10% fetal bovine serum, penicillin at 100 U/mL, streptomycin at 100 µg/mL, and amphotericin B at 1.5 µg/mL at 37℃ and 5% CO2. In this study, only the third to fifth passages were used for experiments. The fibroblasts were incubated with endotoxinfree Alternaria alternate and Aspergillus fumigates at 50 and 25 µg/mL, respectively (Greer Lab, Lenoir, NC, USA). After 8, 24, and 48 hours of stimulation, the cell culture supernatants and cells were harvested and stored at -70℃ until they were assayed.
Immunoreactive TSLP protein was measured in the supernatants using a specific ELISA with matched antibody according to the manufacturer's instructions (R&D system, Minneapolis, MN, USA). The sensitivity limit of the TSLP was 7.8 pg/mL.
TSLP mRNA expression was evaluated with real-time RT-PCR for 40 cycles with denaturation at 95℃ for 15 seconds and annealing/extension at 60℃ for 60 seconds. From the amplified cDNA, the quantitative polymerase chain reaction was performed for TSLP and β-actin in the same 96 well plate using a SYBR® Green PCR core kit (PE Applied Biosystems, Foster City, CA, USA) with the GeneAmp®5700 system (PE Applied Biosystems). The expression levels of TSLP mRNA were normalized by the median expression of β-actin.
The primers used in this study were as follow: TSLP: sense CCC AGG CTA TTC GGA AAC TCA G; antisense CGC CAC AAT CCT TGT AAT TGT G (117 bp), and β-actin: sense ACA GGA AGT CCC TTG CCA TC and antisense AGG GAG ACC AAA AGC CTT CA (248 bp).
After stimulating with the fungi, the cell pellets were placed in cryo-tubes and 1 mL of Trizole reagent was added. The RNA was extracted according to the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany). One microliter of RNA was used for the reverse transcription PCR amplifications and which was performed in a PerkinElmer (Norwalk, CT, USA) thermal cycler. The primers used in this study were as follows: TLR2 (sense, CCA GCA AAT TAC CTG TGT G and antisense, CTG AGC CTC GTC CAT GGG CCA CTC C, 637 bp), TLR3 (sense, CGG GCC AGC TTT CAG GAA CCT G and antisense, GGC ATG AAT TAT ATA TGC TGC, 400 bp), TLR4 (sense, TGC AAT GGA TCA AGG ACC AGA GGC, antisense, GTG CTG GGA CAC CAC AAC AAT CAC C, 449 bp), TLR5 (sense, CCT CAT GAC CAT CCT CAC AGT CAC, antisense, GGC TTC AAG GCA CCA GCC ATC TC, 355 bp), TLR 6 (sense, TGC CCA, TCT GTA AGG AAT TTG, antisense, TGG GTG AAA AAC AAG GTG AAG, 421 bp) and TLR9 (sense, GCG AGA TGA GGA TGC CCT GCC CTA CG, antisense, TTC GGC CGT GGG TCC CTG GCA GAA G, 510 bp). The annealing temperatures were 60℃ for TLR2, 52℃ for TLR3, 61℃ for TLR4, 58℃ for TLR5, 51℃ for TLR6, and 68℃ for TLR9. The samples were visualized using agarose gel electrophoresis and the band densities were measured using the multi Gauge v2.02 (Fujifilm, Tokyo, Japan). The band intensities were expressed as a percentage of treated over untreated cells.
TLR2 and TLR5 mRNA expression was evaluated with realtime RT-PCR. Initial denaturation at 95℃ for 2 minutes, followed by 40 cycles of consisting of denaturation at 94℃ for 10 seconds, annealing at 58℃ for TLR2 and 59℃ for TLR5 for 10 seconds, and elongation at 72℃ for 45 seconds. From the amplified cDNA, the quantitative polymerase chain reaction was performed for TLR2, TLR5 and GAPDH in the same 96 well plate using a SYBR® Green PCR core kit (PE Applied Biosystems) with the GeneAmp®5700 system (PE Applied Biosystems). The expression levels of TLR mRNA were normalized by the median expression of GAPDH. The primers used in this study were as follow: TLR2: sense GTT CCT GCT GAT CCT GCT CAC; antisense CAA ATG CAT CAT AGC AGA TGT TCC(140 bp), TLR5: sense CAG TAT TTG AGG TGG CCT GAG GA; antisense TGC TAC AGT TTG CAA CGG AAT GTT A (126 bp), and GAPDH: sense ACC ACA GTC CAT GCC ATC A; antisense TCC ACC ACC CTG TTG CTG TA (440 bp).
At 70% confluence, the fibroblasts were transfected with siRNA against TLR2, TLR5, or control siRNA at 1 µM using Dharmacon® Accell™ siRNA reagents (Thermo scientific, Hudson, NH, USA). Using 3 different kinds of siRNA (3 target sequence for TLR2; UUC UCA UCU CAC AAA AUU G, CUU GUG ACC GCA AUG GUA U, and UCU UUA UGU CAC UAG UUA U, TLR5; CUC UGA UGC UGU AUU GAA A, CUG GGA AGU AGU AAG AUA U, and CCU UAA AGU CCA UAG AUU U) optimal siRNA was determined with experiments to inhibit the expression of TLR2 and TLR5 mRNA expression from fibroblasts (Fig. 1). The sequences of selected each siRNA were as follows: TLR2: CUU GUG ACC GCA AUG GUA U and TLR5: CUG GGA AGU AGU AAG AUA U. The transfection status of each siRNA was determined with RT-PCR analysis of TLR2 and TLR5 mRNA expression. Transfection was performed according to the manufacture's protocol. In brief, nasal fibroblasts were seeded in wells of a 96-well plate at 1×104 cells/well and incubated at 37℃ and 5% CO2 over night. The siRNA solution was prepared in RNase-free buffered solution and the final concentration of each siRNA was 1 µM per well. After 72 hours, the fibroblasts were stimulated with fungi for 24 hours. The cell culture supernatants and cells were harvested to determine TSLP mRNA expression and protein production.
In normal fibroblasts, TSLP protein production was significantly increased after 24 hours by Alternaria (25 and 50 µg/mL). However, TSLP mRNA expression was not influenced by fungi in normal nasal fibroblasts (Fig. 2).
In nasal polyp fibroblasts, TSLP mRNA expression and protein production were greater than those in inferior turbinate fibroblasts. Alternaria induced TSLP protein production in nasal polyp fibroblasts was increased in a time - and concentration - dependent manner. Alternaria enhanced TSLP mRNA expression in nasal polyp fibroblasts, and the maximal TSLP mRNA expression was noted at 24 hours after stimulation. The effect of Alternaria on the expression of TSLP mRNA and the production of TSLP protein appeared to be stronger than that of Aspergillus. Aspergillus did not significantly influence TSLP mRNA expression or TSLP protein production (Fig. 3).
After 24 and 48 hours of stimulation with Alternaria, TLR2 mRNA expression was significantly increased (1.9 to 2.1 times higher) compared to the non-stimulated group. In addition Aspergillus at 50 µg/mL also enhanced TLR2 mRNA expression in nasal polyp fibroblasts (2.1 times after 48 hours). After 24 and 48 hours of stimulation with 50 µg/mL of Alternaria and 48 hours of stimulation with 50 µg/mL of Aspergillus, TLR5 mRNA expression was significantly increased (Fig. 4). However, TLR3, TLR4, TLR6, and TLR9 mRNA expressions were not influenced by fungal stimulation (data not shown).
Because Alternaria enhanced TLR2 and TLR5 mRNA expressions, nasal polyp fibroblasts were transfected with siRNA against TLR2 and TLR5. When the siRNA-transfected fibroblasts were stimulated with Alternaria for 24 hours, TSLP protein production and TSLP mRNA expression were significantly inhibited by siRNA against TLR2 (about 50% in TSLP protein production and 79% in TSLP mRNA expression) but not with siRNA against TLR5 (Fig. 5).
Fibroblasts are major supporting cells in nasal polyps and can produce various inflammatory mediators. Fibroblasts function as part of the immune system and are initiators of the inflammatory response with several receptors and surface markers that are able to control immune responses.11 Recurrent stimulation of fibroblasts by infectious or inflammatory agents leads to chronic inflammation, and these cells can produce RANTES, VCAM-1, ICAM-1, thymus and activation-regulated chemokine. 711 The proinflammatory cytokines IL-1β and TNF-α induce TLSP production through the NF-κB signal transduction pathway from nasal fibroblasts.7 TSLP is an important cytokine that polarizes CD4+ T cells to Th2 cytokine-producing cells and plays a role as a potent growth and survival factor for Th2 cells. TSLP mRNA was previously found to be highly expressed by cultured skin keratinoyctes, bronchial epithelial cells, smooth muscle cells, and lung fibroblasts.178 In this study, Alternaria enhanced TSLP mRNA expression and TSLP protein production in nasal fibroblasts, especially nasal polyp fibroblasts. TSLP activates dendritic cells, inducing the differentiation of Th2 cytokine-producing CD4+ cells and plays a key role in the development of eosinophilic inflammation in nasal polyps.5 Although Alternaria was able to induce TSLP mRNA expression and TSLP protein production from normal inferior turbinate fibroblasts, nasal polyp fibroblasts more strongly expressed TSLP than normal fibroblasts. This means that nasal polyp fibroblast may be more immunologically active and respond more strongly to pathogenic or environmental stimuli than inferior turbinate fibroblasts. Although inferior turbinate and nasal polyp fibroblasts show similar a immune response, nasal polyp fibroblasts were more strongly response to fungi, so we used nasal polyp fibroblasts for this study.
Fungi have often been associated with bronchial and sinonasal diseases. Airborne fungal spores enter the upper and lower airways by inhalation but are rarely pathogenic in healthy individuals. Some fungal species are associated with airway inflammatory diseases, such as Alternaria, Aspergillus, Cladosporium, Penicillium, and Candida.12 We used Alternaria and Aspergillus to activate nasal fibroblasts because these organisms are known to be common pathogens found in nasal secretions and in respiratory tract diseases.13 Alternaria and Aspergillus extracts activate upper and lower airway epithelial cells with enhancing the production of several inflammatory mediators.89 The interaction of fungi with respiratory epithelial cell receptors, by protease activated receptors (PARs) or toll-like receptors (TLRs), leads to the production of inflammatory chemical mediators, the induction of respiratory bursts, and inflammatory cell recruitment. In bronchial epithelial cells, Alternaria induced TSLP production with the interaction of PAR2. TLRs may not be associated with the production of TSLP.8 Our preliminary experiments with nasal polyp fibroblasts showed that when the cells were stimulated with fungi, Alternaria and Aspergillus enhanced the production of IL-6 and IL-8, but not GM-CSF, and maximal cytokine production was found at the 50 ug/mL treatment of both fungi (data not shown). Therefore, we chose 50 and 25 µg/mL of Alternaria and Aspergillus, respectively, for this study. After stimulating with the fungi, we tried to determine the expression of PAR1, PAR2, and PAR3 mRNA from nasal fibroblasts with RT-PCR. Unlike airway epithelial cells, PARs mRNA expression was not changed by fungal stimulation (data not shown). We then attempted to determine the expression of TLRs and found that TLR2 and TLR5 mRNA expressions were significantly increased by the fungi.
TLRs are essential receptors for the recognition of pathogenic microorganisms and activation of the immune system. The activation of TLRs allows primary defensive immune mechanisms to be initiated locally, which initiate communication of the presence of pathogens to the adaptive immune system.14 Eleven TLR members have been identified in human, and they are triggered by conserved molecular structures expressed by bacteria, virus, and fungi. TLR2, TLR4, and TLR9 are the main TLRs involved in sensing fungal components.15 TLR2 and TLR4 mRNAs have been found to be expressed in nasal epithelial cells and these 2 genes have been reported to be significantly higher in chronic rhinosinusitis.16 Fungal antigens induce the production of IL-6 and IL-12 from dendritic cells via TLR2 and TLR4.16 Alternaria and Aspergillus can enhance TLR2, TLR3, and TLR4 mRNA expression from nasal epithelial cells, and TLR4 is thought to contribute to the production of chemical mediators.9 In nasal fibroblasts, Alternaria and Aspergillus enhanced the expression of TLR2 and TLR5 mRNAs. TLR2 mediates cell responses to lipoproteins and lipoteichoic acid from gram-positive bacteria and some gram-negative bacteria and fungi. TLR2 has been shown to functionally collaborate with distinct types of receptors such as dectin-1, a lectin family receptor for the fungal cell wall component β-glucan.17 TLR5 plays an important role in microbial recognition at the mucosal surface by responses to bacterial flagella and induction of inflammatory cytokine production. However, TLR5 is not commonly associated with fungal infection. Alternaira and Aspergillus enhanced TLR2 and TLR5 mRNA expression by nasal fibroblasts, TSLP protein and mRNA expression were inhibited only with siRNA against TLR2 but not with siRNA against TLR5. Fungi consist of several peptides, enzymes, and other antigenic components, which might be associated with the expression of TLR5. Although both Alternaria and Aspergillus enhanced TLRs mRNA expression, Aspergillus did not induced the production of TSLP. Considering these findings, we need to confirm which component of fungi is associated with the expression of TLR in nasal fibroblasts and the intracellular signal pathway which can induce the production of TSLP. Unlike nasal fibroblasts, synovial fibroblasts release TSLP in response to polyI:C (TLR3 lignad) and LPS (TLR4 lignad).18 Human fibroblasts are not homogenous populations and they express different structural and functional features that are dependent on their location within the body.14 This means fibroblasts from different anatomic sites and pathologic conditions determine characteristics and phenotypes of fibroblasts.
Our findings demonstrate unique characteristics of nasal fibroblasts in which fungi induced TSLP mRNA expression and TSLP protein production from primary nasal fibroblasts. TLR2 is thought to contribute to the production of TSLP. This TSLP can stimulate airway mucosal dendritic cells, which leads to the subsequent development of Th2 immune response in sinonasal mucosa. These immune responses may be involved in the initiation and amplification of CRS with nasal polyps.
Figures and Tables
Fig. 1
Determination of optimal small interfering RNA (siRNA) against toll-like receptors (TLRs) 2 and 5. Fibroblasts were transfected with 3 different kinds of siRNA against TLR2 and TLR5 then stimulated with Alternaria or tumor necrosis factor-α. Second siRNA against TLR2 (target sequence was CUU GUG ACC GCA AUG GUA U) and second siRNA against TLR5 (target sequence was CUG GGA AGU AGU AAG AUA U) most strongly inhibited the expression of TLR2 and TLR5 mRNA from nasal fibroblasts. *P<0.05 compared to Alternaria 50 ug/mL (ALT 50) and TNF-α.
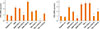
Fig. 2
Effects of fungi on the expression of thymic stromal lymphopoietin (TSLP) from inferior turbinate fibroblasts (A and B) and nasal polyp fibroblasts (C and D). Fibroblasts were stimulated with Alternaria and Aspergillus for 48 hours. When inferior turbinate fibroblasts were stimulated with Alternaria for 24 hours, TSLP protein production was significantly increased (B, P<0.05). However, TSLP mRNA expression was not changed by Alternaria (B). Aspergillus did not influence the expression of TSLP mRNA or the production of TSLP protein from inferior turbinate fibroblasts (A and B). The nasal polyp fibroblasts were stimulated with Alternaria and Aspergillus for 48 hours. When the fibroblasts were stimulated with Alternaria for 24 and 48 hours hours, TSLP mRNA expression and TSLP protein production were significantly increased (C and D, P<0.05). Aspergillus did not influence the expression of TSLP mRNA or the production of TSLP protein from fibroblasts. The values are expressed as the mean±SD of 5 separate experiments. *P<0.05 compared to the negative control. NC, negative control; Alt, Alternaria; Asp, Aspergillus.

Fig. 3
Effects of fungi on the expression of thymic stromal lymphopoietin (TSLP) from nasal polyp fibroblasts. Fibroblasts were stimulated with Alternaria and Aspergillus for 48 hours. When the fibroblasts were stimulated with Alternaria for 24 and 48 hours hours, TSLP mRNA expression and TSLP protein production were significantly increased. Aspergillus did not influence the expression of TSLP mRNA or the production of TSLP protein from fibroblasts. The values are expressed as the mean±SD of five separate experiments. *P<0.05 compared with the negative control. NC, negative control; Alt, Alternaria; Asp, Aspergillus.
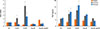
Fig. 4
Quantitative reversee transcription polymerase chain reaction analysis of the expression of toll-like receptors (TLRs) in nasal polyp fibroblasts after simulation with fungi. TLR2 mRNA expression was significantly increased after 24 and 48 hours of stimulation with 50 µg/mL of Alternaria and 48 hours stimulation 50 µg/mL of Aspergillus, and TLR5 mRNA expression was significantly increase after 24 and 48 hours of stimulation with 50 µg/mL of Alternaria and 48 hours stimulation 50 µg/mL of Aspergillus. The values are expressed as the mean±SD of five separate experiments. *P<0.05 compared with the negative control. NC, negative control; Alt, Alternaria; Asp, Aspergillus.
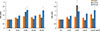
Fig. 5
Effects of siRNA against TLR2 and TLR5 on the expression of thymic stromal lymphopoietin (TSLP) from nasal polyp fibroblasts. Fibroblasts were transfected with siRNA against TLR2, TLR5, or control siRNA for 72 hours then stimulated with Alternaria for 24 hours. TSLP mRNA expression and TSLP protein production were significantly inhibited by siRNA against TLR2. The values are expressed as the mean±SD of five separate experiments. *P<0.05 compared with the non-treated group. NC, negative control; Alt, Alternaria.

References
1. Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007; 293:L375–L382.
2. Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr Opin Immunol. 2010; 22:795–799.
3. Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006; 203:269–273.
4. Ying S, O'Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008; 181:2790–2798.
5. Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011; 3:186–193.
6. Teran LM, Mochizuki M, Bartels J, Valencia EL, Nakajima T, Hirai K, et al. Th1- and Th2-type cytokines regulate the expression and production of eotaxin and RANTES by human lung fibroblasts. Am J Respir Cell Mol Biol. 1999; 20:777–786.
7. Nomura K, Kojima T, Fuchimoto J, Obata K, Keira T, Himi T, et al. Regulation of interleukin-33 and thymic stromal lymphopoietin in human nasal fibroblasts by proinflammatory cytokines. Laryngoscope. 2012; 122:1185–1192.
8. Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009; 183:1427–1434.
9. Shin SH, Lee YH. Airborne fungi induce nasal polyp epithelial cell activation and toll-like receptor expression. Int Arch Allergy Immunol. 2010; 153:46–52.
10. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.
11. Fukumoto A, Nonaka M, Ogihara N, Pawankar R. Induction of TARC production by lipopolysaccharide and interleukin-4 in nasal fibroblasts. Int Arch Allergy Immunol. 2008; 145:291–297.
12. Shin SH, Ye MK, Kim JK. Effects of fungi and eosinophils on mucin gene expression in rhinovirus-infected nasal epithelial cells. Allergy Asthma Immunol Res. 2014; 6:149–155.
13. Shin SH, Ye MK, Lee YH. Fungus culture of the nasal secretion of chronic rhinosinusitis patients: seasonal variations in Daegu, Korea. Am J Rhinol. 2007; 21:556–559.
14. Janssens S, Beyaert R. Role of toll-like receptors in pathogen recognition. Clin Microbiol Rev. 2003; 16:637–646.
15. Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Tolllike receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007; 7:1271–1285.
16. Dong Z, Yang Z, Wang C. Expression of TLR2 and TLR4 messenger RNA in the epithelial cells of the nasal airway. Am J Rhinol. 2005; 19:236–239.
17. Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003; 197:1107–1117.
18. Ozawa T, Koyama K, Ando T, Ohnuma Y, Hatsushika K, Ohba T, et al. Thymic stromal lymphopoietin secretion of synovial fibroblasts is positively and negatively regulated by Toll-like receptors/nuclear factor-κB pathway and interferon-γ/dexamethasone. Mod Rheumatol. 2007; 17:459–463.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download