Abstract
Purpose
Clinical features of peanut allergy can range from localized to systemic reactions. Because peanut and birch pollen have cross-reactivity, peanut can lead to localized allergic reaction in Fagales pollen-sensitized oral allergy syndrome (OAS) patients without peanut sensitization per se. The purpose of this study was to discriminate true peanut food allergy from cross-reactive hypersensitivity in birch-sensitized peanut allergy.
Methods
Birch-sensitized (n=81) and peanut anaphylaxis patients (n=12) were enrolled. Peanut-related allergic reactions and sensitization profiles were examined. Specific IgE to Fagales tree pollens (birch, oak), peanut, and their component allergens (Bet v 1, Bet v 2, Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9) were evaluated. Based on these specific IgEs and clinical features, the patients were classified into 4 groups: group 1 (Fagales pollen allergy without OAS), group 2 (Fagales pollen allergy with OAS), group 3 (OAS with peanut anaphylaxis), and group 4 (peanut anaphylaxis).
Results
After peanut consumption, one-third of OAS patients experienced oral symptoms not associated with peanut sensitization. Ara h 1 or Ara h 2 was positive in peanut anaphylaxis patients, whereas Ara h 8 was positive in OAS patients. There were 4 patients with both peanut anaphylaxis and OAS (group 3). Both Ara h 2 and Ara h 8 were positive in these patients. Foods associated with OAS in Korea showed unique patterns compared to Westernized countries.
Peanut allergy patients have diverse clinical manifestations, depending on age, ethnicity, and sensitization profiles. In adults, sensitization and clinical patterns of peanut allergy are different from those in children.12 Peanut anaphylaxis (PA) is common in children; oral allergy syndrome (OAS) to peanut is common in adults. Cross-reactivity between Fagales tree (including birch and oak family trees) pollen and peanut can explain the age difference observed in peanut allergy, as the Fagales pollen sensitization rate increases with age.3 As some patients have both PA and OAS, accurate diagnosis of peanut allergy and prediction of prognosis are important for these patients.
In Asian populations, peanut allergic patients are rare compared to those observed in Western countries.4 However, the incidence of peanut allergy among Asian populations also continues to increase, though the precise cause of this phenomenon is currently unknown.56 The increasing incidence of peanut allergy in Korea may be due to increased Fagales sensitization, which is cross-reactive to peanuts. The number of tree pollen-sensitized patients is increasing worldwide due to changes in atmospheric CO2, climate, and pollen counts;7 the incidence of Fagales pollen-related OAS is also increasing.8 In Korea, Fagales pollen sensitization is also increasing, which may be due to climate change and restoration of forests after the Korean War and the consecutive period of industrialization.
Differences in the sensitization to major peanut allergens have been reported in many previous studies. The Ara h 2 component allergen is a storage protein in peanuts and well known to be suitable for diagnosing PA.9 However, OAS patients can experience oral symptoms after peanut consumption; these cases are usually associated with Ara h 8, which belongs to the pathogenesis-related protein (PR)-10 family. The PR-10 family includes Bet v 1, Que a 1, Aln g 1, and Cor a 1. Furthermore, the sensitization patterns of peanut component allergens also differ among countries.10
The aim of this study was to identify different sensitization patterns of birch and peanut component allergens associated with PA and OAS. These differences may enable physicians to accurately diagnose patients and to implement appropriate treatment plans for peanut allergy. In addition, diets and eating habits differ between Western and Eastern countries, and therefore, it is necessary to determine the patterns of OAS in East Asian countries.
Birch pollen allergic patients (n=81) and PA patients (n=12) were retrospectively enrolled from January 2013 to June 2015 from the Severance Resource Allergy Data System (Seoul, Korea), which includes clinical history, diagnosis, and sensitization profiles of patients' allergic diseases with their sera. Sensitization was determined using ImmunoCAP and skin prick test (SPT) results. Patients' medical records were retrospectively reviewed for detailed clinical symptoms of cross-reactivity of food allergens to sensitized tree pollens.11 If the exact symptoms were not recorded, we conducted telephone interviews with the patients to obtain a more detailed clinical history. Based on the sensitization profiles and clinical manifestations, the patients were categorized into 4 groups. Group 1 included birch pollen allergic patients without OAS (patients with allergic rhinitis or asthma); group 2 included birch pollen-related OAS patients; group 3 included patients with both OAS and PA; and group 4 included patients with PA but without OAS. Diagnosis of OAS and PA were determined by careful history taking, symptom reproducibility and sIgE titers. The classification of each group is shown schematically in Fig. 1. Sensitization profiles for birch and peanut were analyzed including component allergens. This study was approved by the Institutional Review Board of Yonsei University Health System (4-2013-0397). Participating patients provided written informed consent.
SPTs were performed on the patients' backs using 55 kinds of allergens, including trees, grasses, weeds, molds, house dust mites, animal dander, white birch pollen, and white oak tree pollen (Allergopharma, Hamburg, Germany); a negative control (normal saline with 0.3% phenol and 50% glycerol) and positive control (0.1% histamine; Allergy Therapeutics, Worthing, UK) were also tested. The SPT results were interpreted as positive if the wheal size of each allergen averaged ≥3 mm in diameter.
Sera of the enrolled subjects, which were stored at -70℃, were used for IgE measurement. The ImmunoCAP system (Thermo Fisher, Uppsala, Sweden) was used to measure specific IgE (sIgE) to peanut and tree pollens. The total allergen and 5 recombinant component allergens (Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9) of peanuts were measured using the serum of each patient; sIgE titers ≥0.35 kU/L were considered positive. Titers >100 kU/L were regarded as 101 kU/L for statistical analysis. The levels of sIgE against the total birch and oak tree pollens and component allergens of birch (Bet v 1, Bet v 2) were measured.
The data were analyzed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Patients' baseline characteristics were analyzed using Fisher's exact, Pearson's χ2, and Kruskal–Wallis tests. The positivity rate for each allergen was compared using Fisher's exact or Pearson's χ2 tests. The sIgE titers were compared with Mann-Whitney U and Kruskal-Wallis tests. Dunn's test was used for subgroup comparisons after the Kruskal-Wallis test. Receiver operating characteristic (ROC) curve analysis was performed to determine the sIgE cut-off titers for OAS. A comparison of the ROC curve was performed using MedCalc software (MedCalc, Mariakerke, Belgium). A P value of <0.05 was considered statistically significant.
The demographic characteristics of the enrolled patients are shown in Table 1. The mean age of the patients was 30.3 years. In Korea, PA in adults is rare, so that the mean age of group 4 was 7.6 years. All the food allergy patients (groups 2, 3, and 4) re-experienced immediate allergic reactions by culprit food allergen. The sex ratio was not different between groups. Total birch- sIgE level was highest in the OAS group (P=0.004), which was 2 times higher than in the non-OAS group. Initially, we speculated that group 4 patients were not sensitized to birch. However, there were birch-sensitized patients (n=5, 62.5%) in group 4 as determined by the ImmunoCAP test. The total peanut-sIgE level was highest in group 4 (P<0.001) and 3 times higher than in group 3. Clinical diagnosis and manifestations are also shown in Table 1.
In the OAS group (groups 2 and 3), apple was the most common cause of OAS, followed by peach, plum, and cherry in the Rosaceae family. Regarding the Fabaceae family, 18 patients (36.0%) experienced allergic reactions to peanuts, and 11 patients (22.0%) had reactions to legumes. With peanuts, 4 patients experienced anaphylaxis, while the others (n=14) experienced only localized reactions. Four patients with both OAS and PA (group 3) experienced anaphylaxis after peanut consumption, with localized oral symptoms after eating apples or peaches. The symptoms of OAS were limited to the mouth and oropharynx, and included an itching sensation, lip swelling, and erythematous changes. Patients commonly complained that the types of fruits that they were able to consume decreased with time.
Interestingly, culprit foods associated with OAS in Korea are somewhat different from those in Westernized countries. We found a prevalence of OAS in patients with apple allergy that was similar to that reported in a previous study.12 However, none of the patients in our study had hazelnut allergy, which is not a popular food in Korea. Interestingly, 7 patients (14.0%) experienced oral symptoms after eating fresh Korean ginseng, a popular herbal medicine in Asia. Cherry, kiwi, chestnut, and ginseng showed similar allergic frequencies in the OAS group. The allergic response rate for different food types in OAS patients is shown in Table 2.
The positivity rates of peanut and its component allergens are shown in Fig. 2A. The positive rate of the allergens was significantly different between the groups. The positive rate of total peanut sIgE was 100% in the PA group (groups 3 and 4). In the OAS group, total peanut sIgE positivity was not correlated with symptoms. Interestingly, 19.2% of patients who had clinical symptoms to peanuts had a negative reaction to total peanut allergens.
The positivity rate to anaphylaxis-related component allergens (Ara h 1, Ara h 2, or Ara h 3) was 100% in group 4. In group 4 patients, 7 out of 8 showed a positive response to Ara h 2. The remaining patient showed a positive response only to Ara h 1. Only 1 patient (1/12, 8.3%) in the PA group (groups 3 and 4) had no reaction to anaphylaxis-related component peanut allergens. In the non-anaphylaxis group (group 1 and 2), there were 3 (3.7%) patients who showed a positive response to Ara h 1, Ara h 2, or Ara h 3. Ara h 8 was highly positive in the OAS group (groups 2 or 3). In group 3, Ara h 2 showed 75% (3/4) positivity, and Ara h 8 showed 100% positivity. However, Ara h 8 positivity did not correlate with symptoms to peanut.
The sIgE titers of the 4 subgroups were also compared, and the results are shown in Fig. 2B. The total peanut sIgE titers were higher in the PA group than in the non-PA group. Similar patterns were found for Ara h 1, 2, and 3. These differences in total peanut allergens, Ara h 1, Ara h 2, and Ara h 3 were statistically significant. In contrast, Ara h 8-sIgE titers were highest in group 3, but there was no statistical significance. As mentioned earlier, 62.5% of group 4 patients were unexpectedly regarded as birch sensitizer. Ara h 9 was highly positive in group 3, but the titers were not significantly different between the groups. The mean titers of Ara h 9 in group 3 was 0.26±0.19 kU/L
To demonstrate the sensitization profiles more clearly according to the clinical manifestation, patients were divided into 2 groups, depending on whether the symptom for peanut was OAS or PA. IgE positivity and titers are shown in Fig. 3A and B. PA patients showed the highest response to Ara h 2 (83.3%). Specific IgE titers to peanut, Ara h 1, Ara h 2, and Ara h 3 were higher in the anaphylaxis group than in the localized symptom group (P<0.001). Patients who had localized reactions to peanut showed high positivity to Ara h 8. However, 75.0% (9/12) of patients in the PA group (groups 3 and 4) were also sensitized to birch pollen and 50.0% (6/12) to Ara h 8. For this reason, mean sIgE titers of Ara h 8 showed subtle difference between the groups (P=0.231). The positive rates of Ara h 3 and Ara h 9 were not significantly different between the groups in the Korean population. The sIgE titers of Ara h 9 was statistically different (Fig. 3B). However, the mean titers were 0.12 kU/L in the localized symptom group and 0.21 kU/L in the PA group.
Among all the participants, 63 (67.7%) underwent SPT. Among these participants, 82.5% and 82.5% were sensitized to birch and oak pollens, respectively. Alder pollen sensitization (86.7%) was found to have a pattern similar to that of birch and oak, and is known to be cross-reactive to birch tree pollen.13 When sensitization was based on ImmunoCAP results, which was performed iw n 90 patients (96.8%), 98.9% and 97.8% of the patients were sensitized to both birch and oak pollen, respectively. The percentages of positive responses to birch component allergens were 82.0% and 19.7% for Bet v 1 and Bet v 2, respectively.
In order to show the sensitization patterns more specifically, birch-sensitized patients were divided into 2 groups: the non-OAS group (groups 1 and 4) and the OAS group (groups 2 and 3). Sensitization patterns are shown in Fig. 4. The sIgE titers of birch, oak, Bet v 1, and Ara h 8 were significantly higher in the OAS group than in the non-OAS group. Birch-sIgE titers of OAS patients were 1.7 times higher than thoseof non OAS patients. The sIgE titer of Bet v 1 was 2.4 times higher in OAS patients compared to nonOAS patients. The 2 component allergens Bet v 1 and Ara h 8 are belonged to the PR-10 family, and agreement of the allergens was 93.8%. The sIgE titers of Bet v 2 were not different between the 2 groups, regardless of the presence of OAS symptoms.
To predict OAS using sIgE titers, the ROC curve and optimal cutoff sIgE titer are shown in Fig. 5. Predictive abilities of OAS were not different between the sIgE titers for birch pollen, Bet v 1, and Ara h 8.
In this study, the sensitization patterns and clinical symptoms of patients with peanut and birch allergy were evaluated. Since tree pollen counts have increased over the past 15 years in Korea, the oak and birch tree sensitization rates have increased from 4.4% to 14.4%, and from 7.1% to 13.6%, respectively.14 Although birch is not a dominant species in South Korea, there are many cross-reactive Fagaceae and Betulaceae family trees, including the Quercus and Alnus species.15 In Korea, Pinales (pine trees) and Fagales trees make up 80% of forests. Pine tree pollens are the most common in Korea, accounting for 70% of all tree pollens.16 Dominant Fagales trees in Korea include Quercus mongolica, Quercus serrata, Quercus aliena, and Quercus acutissima. Choi et al.17 reported that as the temperature rises, so does the proportion of Quercus species. Climate changes in Korea exceed the global climate change rate, especially with regard to temperature changes.18 Patients sensitized to tree pollen and those with OAS in Korea will be increasing due to this climate trend.
Similar to Western countries, the incidence rates of peanut allergy and sensitization are increasing in Korea. In this study, 57.1% of patients in Korea with tree pollen allergy had total peanut sIgE, regardless of symptoms. It is difficult to confirm peanut allergy using the total peanut sIgE test. In this study, 19.2% of patients who exhibited symptoms in response to peanuts had a negative reaction to total peanut allergens. Total peanut sIgE is not enough to diagnose cross-reactive peanut allergy in those sensitized to Fagales.
Sensitization profiles of peanut component allergens can be used for precise risk assessments and prediction of symptom severity. There have been many previous studies regarding recombinant peanut component allergens. When patients are cosensitized to Ara h 1, 2, and/or 3, they tend to experience severe allergic reactions to peanuts.1920 Similar to a previous peanut component study, this study showed that Ara h 2 was positive in patients with PA, and that Ara h 8 was positive in those with localized symptoms. In patients with both PA and OAS, both Ara h 2 and Ara h 8 were positive. Among 12 PA patients, there was 1 patient (8.3%) who was only sensitized to Ara h 8 without Ara h 1, Ara h 2, or Ara h 3. (The patient was included in group 3.) In a previous Korean study, Ara h 2 was less prevalent than in Western countries.21 Large-scale research is needed to understand PA sensitization patterns in Korean patients. Based on our data, we were unable to clarify the order of sensitization between birch and peanut. Ara h 9, which belongs to a lipid transfer protein, has limited clinical significance in Korea, but it is the most frequently sensitized peanut allergen in Mediterranean countries (i.e., Spain), which suggests the possibility of cross-reactivity between the group 3 peach major allergen (Pru p 3) and Ara h 9.22
Peanut component-resolved diagnosis can be used to discriminate the cross-reaction to birch pollen and predict the severity of peanut allergy.23 This precise diagnosis can lead to decreased unnecessary food elimination and medical costs. It may also play a role in discriminating between OAS patients with peanut allergy that may be treated by pollen-specific immunotherapy and patients with immunotherapy-intractable peanut allergies. According to our ROC evaluation, a cutoff value for birch or Bet v 1 can be applied to determine appropriate initiation timing of allergen immunotherapy.
The sensitization patterns of Bet v 1 and 2 vary among countries.24 According to a previous study in Korea, the sensitization rates for Bet v 1 and 2 confirmed by an immunoblot were 78.9% and 75.8%, respectively.13 In this study, 82.0% and 19.7% of patients sensitized to birch pollen were Bet v 1- and v 2-positive, respectively. This difference can be explained by the use of different birch species and detection techniques. According to this study, Bet v 1 had more clinical implications compared to Bet v 2. A higher titer of Bet v 1 better correlates with OAS symptoms.
In addition, differences in the allergic response rate of various fruits in OAS patients should also be mentioned. Cross-reactive foods with tree pollen were somewhat different from that in Western countries. Apples or peaches were the most frequent causes of OAS in Korea. However, there were patients allergic to chestnuts and Korean ginseng. Ginseng is generally used as an herbal medicine in Asian countries. Currently, there is only 1 published study about the cross-reactivity between Korean ginseng and birch pollens.25 Ginseng is available in fresh or dried forms and can be added to teas, alcoholic drinks, energy drinks, or skin cosmetics. Furthermore, it can be used for intravenous injection and acupuncture as a type of alternative medicine. When used as alternative medicine, patients can experience severe allergic reactions after ginseng treatment. There have been 2 case reports regarding ginseng-induced asthma and anaphylaxis; both patients were sensitized to the pollen of birch and alder trees.2627 Clinicians should consider ginseng as a possible culprit for OAS. Chestnut allergy is common in latex-fruit allergy patients.28 The biochemical classification of typical OAS (usually PR-10 related birch apple syndrome) is different from latex-fruit allergy (usually PR-3 related).29 Sensitization to profilin (Bet v 2) has also been recognized to be important in the pathogenesis of OAS;2230 however, Bet v 2 positivity was not correlated with clinical symptoms in our study.
This study has certain limitations, namely its retrospective design. We could not directly compare changes in pollen count, sensitization rate, and peanut allergy incidence. In addition, the number of patients with PA was small. Furthermore, this research was based on mainly adult patients. As the number of pediatric OAS patients is also increasing in Korea, additional research including pediatric patients is needed. Further studies are needed to evaluate the efficacy of allergen immunotherapy using tree pollens as a treatment for peanut-related OAS patients. In some subgroups, patients with peanut allergy can be treated using immunotherapy.
In conclusion, the increase in Fagales pollen allergy can influence the prevalence of peanut allergies. To discriminate between OAS involving peanut from vignette peanut allergy in Korea, it is crucial to measure component peanut allergens, especially Ara h 2 and Ara h 8.
Figures and Tables
Fig. 2
Sensitization profiles of peanut allergens. (A) Positive rates, (B) Specific (IgE titers to total and component peanut allergens.
*P value<0.05; **P value<0.005.

Fig. 3
Sensitization profiles of peanut allergens in peanut allergic patients. (A) Positive rates, (B) Specific IgE titers to total and component peanut allergens in patients with peanut allergy.
*P value<0.05; **P value<0.005.

Fig. 4
Specific IgE titers to total and component tree pollen allergens in patients with and without oral allergy syndrome (OAS).
*P value<0.05; **P value<0.005.
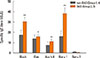
Fig. 5
Receiver operating characteristic (ROC) curve of specific sIgE titers for prediction of oral allergy syndrome (OAS).
AUC, area under the curve; CI, confidence interval.

Table 1
Baseline characteristics of the patients

Table 2
Allergic response rates for each food in patients with oral allergy syndrome (n=50)

ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI 13C0010).
References
1. Ballmer-Weber BK, Lidholm J, Fernández-Rivas M, Seneviratne S, Hanschmann KM, Vogel L, et al. IgE recognition patterns in peanut allergy are age dependent: perspectives of the EuroPrevall study. Allergy. 2015; 70:391–407.
2. Garcia-Blanca A, Aranda A, Blanca-Lopez N, Perez D, Gomez F, Mayorga C, et al. Influence of age on IgE response in peanut-allergic children and adolescents from the Mediterranean area. Pediatr Allergy Immunol. 2015; 26:497–502.
3. Schmitz R, Ellert U, Kalcklösch M, Dahm S, Thamm M. Patterns of sensitization to inhalant and food allergens - findings from the German Health Interview and Examination Survey for Children and Adolescents. Int Arch Allergy Immunol. 2013; 162:263–270.
4. Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013; 3:3–14.
5. Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007; 120:491–503.
6. Chiang WC, Kidon MI, Liew WK, Goh A, Tang JP, Chay OM. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007; 37:1055–1061.
7. Bajin MD, Cingi C, Oghan F, Gurbuz MK. Global warming and allergy in Asia Minor. Eur Arch Otorhinolaryngol. 2013; 270:27–31.
8. Kim SH, Park HS, Jang JY. Impact of meteorological variation on hospital visits of patients with tree pollen allergy. BMC Public Health. 2011; 11:890.
9. Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC, et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011; 154:216–226.
10. Vereda A, van Hage M, Ahlstedt S, Ibañez MD, Cuesta-Herranz J, van Odijk J, et al. Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011; 127:603–607.
11. Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001; 108:881–890.
12. Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, Greiderer A, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011; 127:616–622.e1.
13. Yoon MG, Kim MA, Jin HJ, Shin YS, Park HS. Identification of immunoglobulin E binding components of two major tree pollens, birch and alder. Allergy Asthma Respir Dis. 2013; 1:216–220.
14. Park HJ, Lim HS, Park KH, Lee JH, Park JW, Hong CS. Changes in allergen sensitization over the last 30 years in Korea respiratory allergic patients: a single-center. Allergy Asthma Immunol Res. 2014; 6:434–443.
15. Weber RW. Patterns of pollen cross-allergenicity. J Allergy Clin Immunol. 2003; 112:229–239.
16. Oh JW, Lee HB, Kang IJ, Kim SW, Park KS, Kook MH, et al. The revised edition of Korean calendar for allergenic pollens. Allergy Asthma Immunol Res. 2012; 4:5–11.
17. Choi K, Kim M, Lee WK, Gang HU, Chung DJ, Ko EJ, et al. Estimating radial growth response of major tree species using climatic and topographic condition in South Korea. J Clim Chang Res. 2014; 5:127–137.
18. Cho KT, Jeong HM, Han YS, Lee SH. Variation of ecological niche of quercus serrata under elevated CO2 concentration and temperature. Korean J Environ Biol. 2014; 32:95–101.
19. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010; 125:191–197.e1-13.
20. Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, et al. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006; 118:250–256.
21. Kim J, Lee JY, Han Y, Ahn K. Significance of Ara h 2 in clinical reactivity and effect of cooking methods on allergenicity. Ann Allergy Asthma Immunol. 2013; 110:34–38.
22. Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, et al. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009; 124:771–778.e5.
23. Ferreira F, Wolf M, Wallner M. Molecular approach to allergy diagnosis and therapy. Yonsei Med J. 2014; 55:839–852.
24. Movérare R, Westritschnig K, Svensson M, Hayek B, Bende M, Pauli G, et al. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol. 2002; 128:325–335.
25. Kim M, Choi E, Yoon T. Korean ginseng makes oral allergy syndrome in birch-sensitized respiratory allergy patients. J Allergy Clin Immunol. 2008; 121:Suppl 1. S187.
26. Lee JY, Lee YD, Bahn JW, Park HS. A case of occupational asthma and rhinitis caused by Sanyak and Korean ginseng dusts. Allergy. 2006; 61:392–393.
27. Lee JY, Jin HJ, Park JW, Jung SK, Jang JY, Park HS. A case of Korean ginseng-induced anaphylaxis confirmed by open oral challenge and basophil activation test. Allergy Asthma Immunol Res. 2012; 4:110–111.
28. Blanco C. Latex-fruit syndrome. Curr Allergy Asthma Rep. 2003; 3:47–53.
29. Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000; 106:27–36.
30. Sekerková A, Poláčková M. Detection of Bet v1, Bet v2 and Bet v4 specific IgE antibodies in the sera of children and adult patients allergic to birch pollen: evaluation of different IgE reactivity profiles depending on age and local sensitization. Int Arch Allergy Immunol. 2011; 154:278–285.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download