Abstract
Purpose
The incidence of allergen specific immunotherapy-related systemic reactions (SRs) varies among different studies, and many factors are likely to contribute to SRs. This study aims to investigate the incidence, characteristics, and risk factors of SRs to standardize dust mite-specific subcutaneous immunotherapy (SCIT) in Central China.
Methods
All patients receiving standardized dust mites (100-100,000 SQ-U/mL; Alutard SQ, Hørsholn, Denmark) immunotherapy were followed up. Recorded data included demographics, diagnosis, patient status, pulmonary function testing results before and after each injection, allergen dosage, and details of SRs.
Results
From June 2011 to August 2014, a total of 208 patients received 4,369 injections; 27 (13.0%) patients experienced 48 (1.1%) systemic reactions. Most of the SRs were grade 2 reactions (n=30, 62.5%), followed by grade 1 (n=11, 22.9%), grade 3 (n=7, 14.6%), and no fatal reactions occurred. Forty-six SRs (95.8%) occurred within 30 minutes. Higher SR rates were associated with high concentration extracts (100,000 SQ-U/mL), injections with concomitant local reactions (LRs), children, asthma and high sensitivity (skin prick test 3+/4+ and/or sIgE≥17.5 kUA/L) (P<0.05). The estimated odds of SRs increased in children (OR=6.57; 95% CI: 1.88-22.97, P=0.003), asthmatic patients (OR=4.10; 95% CI: 1.72-9.80, P=0.002), and injections with LRs (OR=2.41; 95% CI: 1.33-4.36, P=0.004).
Subcutaneous allergen immunotherapy (SCIT) is confirmed with significant benefit in properly selected patients with allergic rhinitis and/or asthma.1 However, SCIT is associated with risk of systemic reaction (SR), which may be severe, even life-threatening. Concerns about the safety and convenience of SCIT limit the application of this effective therapy. Identification of specific risk factors for SRs would be helpful in pharmacovigilance and framing a discussion about SCIT risk with patients. Several risk factors, including type of extracts, administration errors, build-up dosage, rush therapy or cluster therapy, asthma, seasonal exacerbation of symptoms, prior systemic reaction, and use of beta blockers and angiotensin-converting enzyme (ACE) inhibitors, have been evaluated in previous studies, while there still exist inconsistent findings.23 Despite identification of these risk factors, the reported incidence of SCIT-related nonfatal SRs has not changed remarkably in over 20 years, which implies the occurrence of SRs depends on a number of factors and may vary in different regions and countries. The results obtained in a single center cannot be easily extrapolated to all countries and realities. Therefore, this study was undertaken to investigate the incidence, characteristics and risk factors of SRs in patients treated with standardized dust mite (Dermatophagoides pteronyssinus [Dp]) SCIT for respiratory allergies in Central China.
The study was conducted in Tongji Hospital. A total of 208 patients from 9 cities in Central China who received SCIT from June 2011 to August 2014 were enrolled in our study. The study was approved by the Independent Ethical Committee of Tongji Hospital, and each participator or his/her statutory guardian signed the informed consent of the immunotherapy.
Patients enrolled in our study were those who: (1) were diagnosed with allergic rhinitis and allergic asthma with or without rhinitis according to the ARIA and GINA guidelines, (2) showed positive skin prick tests to Dp and serum Dp-specific IgE levels ≥ grade 2, and (3) had allergic symptoms of rhinitis and/or asthma after exposure to Dp. On the other hand, patients with autoimmune diseases, cardiovascular disorders, and use of beta blockers or ACE inhibitors were excluded from SCIT.
Skin tests for 19 kinds of inhalant allergens were performed, including dust mites (Dp and Dermatophagoides farina [DF]), cockroach, mulberry silk, animal dander (cat, dog, sheep, and horse), tree pollens (Sabina, Platanus, Populus, and cryptomeria), weed pollens (Artemisia, Ambrosia, and Humulus), and fungi (Alternaria, Cladosporium, Aspergillus, and Paecilomyces) (Macro-Union Pharmaceutical, Beijing, China). Histamine (10 mg/mL) and diluent were used as positive and negative controls. Serum specific IgEs were measured by ImmunoCAP (Phadia, Thermo Fisher Scientific, Uppsala, Sweden).
Sensitization to dust mites was defined by typical history combined with positive skin prick tests (SPTs) and serum specific IgE measurements. A positive skin reaction was defined as a wheal size of ≥3 mm, after subtraction of the negative control. Skin index (SI) was calculated by the ratio of wheal diameter of each allergen to diameter of wheal induced by histamine and scaled by the following criteria: SI <0.5, "1+"; 0.5≤ SI <1.0, "2+"; 1.0 ≤ SI <2.0, "3+"; SI ≥2.0, "4+".4 The cutoff value of serum specific IgE was 0.35 kUA/L, with the grading of positive measurements as follows: grade 1 ≥0.35 kUA/L, grade 2 ≥0.7 kUA/L, grade 3 ≥3.5 kUA/L, grade 4 ≥17.5 kUA/L, grade 5 ≥50 kUA/L, and grade 6 ≥100 kUA/L. The 3+/4+ SPT reactions and/or specific IgE measurements greater than grade 4 were defined as greatly sensitized reactivity or high sensitization.
All the patients received allergen injection in Tongji Hospital. Standardized Dp allergen extracts with depot formulations (Alutard SQ, Horsholm, Denmark) were used for SCIT. There were 4 different vials of standardized allergen extracts, No. 1-4 vial, in which allergen concentration increased by 10-fold from 100 to 100,000 SQ-U/mL. The build-up phase was carried out with the conventional schedule provided by the manufacturer, with weekly injections by the volume of 0.2, 0.4, and 0.8 mL in No. 1 to 3 vial and 0.1, 0.2, 0.4, 0.8, and 1.0 mL in No. 4 vial, reaching the maintenance dose, 100,000 SQ-U. Then, the maintenance dose was given on a 6-week basis according to the manufacturer's instructions. The dose was reduced in the condition such as injections following the last SR and prolonged interval between 2 consecutive injections (>8 weeks). Pulmonary function testing was performed before and after each injection. The injection would be delayed if pre-injection forced expiratory volume in 1 second (FEV1) and/or peak expiratory flow (PEF) were below 80% of the predicted value. After each injection, patients were kept under observation for 30 minutes by nurses and/or physicians. Allergen extract dosage, adjustment in therapy, pulmonary function testing results, and details of SRs, such as onset time, manifestations, and treatment, were recorded.
A local reaction (LR) refers to any symptom or sign located at or nearby the injection site and usually manifests as redness, pruritus, and swelling, of which the maximum diameter greater than 25 mm is defined as a large local reaction (LLR);5 a systemic reaction (SR) is defined as an adverse reaction involving organ-specific systems distant from the injection site. The severity of SRs is classified into 5 grades according to the World Allergy Organization (WAO) SCIT SR grading system.6 A reaction from a single organ system, such as cutaneous, conjunctival, or upper respiratory, but not asthma, gastrointestinal, or cardiovascular is classified as grade 1. Symptoms/signs from more than 1 organ system, asthma (less than 40% PEF or FEV1 drop, responding to an inhaled bronchodilator), or gastrointestinal symptoms/signs are classified as grades 2; either asthma (more than 40% PEF or FEV1 drop, not responding to an inhaled bronchodilator) or laryngeal/uvula/tongue edema with or without stridor as grade 3. Respiratory failure or hypotension, with or without loss of consciousness, was defined as grade 4, and death as grade 5.
Descriptive parameters, such as means and standard deviations for normally distributed continuous data, median percentiles for non-normally distributed continuous data, and frequencies and percentages for categorical data, were calculated. Nonparametric equivalent Wilcoxon rank-sum tests or 2-sample t tests were used to evaluate the association between SR and continuous measures. Pearson's χ2 tests (Yates corrected χ2 if necessary) or Fisher's exact tests were used to evaluate the association between SR and categorical measures. Odds ratios (ORs) were calculated between groups, with 95% confidence intervals generated. For multivariable analysis, logistic regression with forward model selection and likelihood ratio test was applied to assess the predictive model of the dependent variable SR. All tests were performed 2-tailed, and a probability value of less than 0.05 was considered statistically significant. SPSS software (version 19.0, IBM) was used for all statistical analyses.
A total of 208 patients (80 females and 128 males) were included in this study. Their mean and median ages were 19.2 and 12.5 respectively, ranging from 4 to 65 years, and there were 121 children (<18 years) and 87 adults (≥18 years). The diseases included were allergic rhinitis (140, 67.3%), asthma (22, 10.6%), and allergic rhinitis complicated with asthma (46, 22.1%). Among the patients, 178 (85.6%) were mono-sensitized to dust mites, and 30 (14.4%) were multi-sensitized. There were more greatly sensitized subjects in the children group (Table 1). A total number of 4,369 SCIT injections were administered.
Twenty-seven patients (13.0%) developed 48 systemic reactions (1.1% of injections). Nine (one-third) patients experienced more than 1 SR, and 43.8% (21/48) of the SRs occurred with at least 1 previous SR.
Symptoms/signs of the SRs included were either generalized pruritus and urticaria, conjunctival pruritus, rhinitis symptoms/signs (nasal pruritus, sneezing, rhinorrhea, and nasal congestion), itchy throat, asthma symptoms/signs (shortness of breath, cough, and wheezing), or declines in PEF or FEV1. No fatal reaction occurred. According to the WAO SCIT SR grading system, the severity of SRs ranged between grades 1 and 3 (Table 2).
Most (46/48, 95.8%) of the SRs occurred within 30 minutes of the injection, with the earliest onset of 10 minutes. Only 2 SRs were delayed reactions, of which one manifested as facial edema and chest tightness after 3 hours and the other manifested as asthma after 8 hours.
Patients with SRs had a satisfactory response to treatment with medications, such as oral H1 antihistamines, inhaled β2 agonists, intramuscular corticosteroids, and epinephrine (Table 3). As for the 2 patients with delayed reactions, one with facial edema and chest tightness was managed with symptomatic treatment in the community hospital, and the other with asthma was relieved after using inhaled β2 agonist at home.
Injection doses in patients who experienced an SR were downgraded 1 step or reduced 0.2 mL at the next injection visit, according to the extracts product instructions, and then gradually increased to the maintenance dose. However, the maintenance dose of 16 children and 2 adults did not reach the maximum (100,000 SQ-U), which accounted for two-thirds of the patients with SRs, and the proportion of patients whose maintenance dose did not reach the maximum was much higher in children than in adults (13.22% vs 2.30%, P=0.006). There were 2 dropouts in the children group.
Most (44/48, 91.7%) of the SRs occurred during injecting of No. 4 vial, and the SR rate was much higher with No. 4 vials than that with lower concentration extracts (Fig. 1). There was no significant difference in the SR rates of No.4 vials between build-up and maintenance phases (1.71% vs 1.78%, P=0.9), which was the same in children subgroups (2.87% vs 2.93%, P=0.9).
Patients with a history of LRs had a higher SR rate than those with no history of LRs, but the difference was not statistically significant (13.86% vs 9.52%, P=0.456). However, the SR rate was higher in injections with immediately preceding LR or LLR than those without (LR: 2.07% vs 0.73%, P<0.001; LLR: 5.45% vs 1.04%, P=0.022).
The median age of patients with SRs was 7 years, which was much younger than who never experienced SR (Fig. 2). The SR rates of different subgroups divided according to sex, age, diseases, and sensitization status (mono-/multi-sensitized, sensitization degree) are shown in Table 4. There were higher SR rates in children in the presence of asthma or greatly sensitized status. SR rates of 121 children patients between different subgroups were further analyzed (Table 5).
Multivariate logistic regression demonstrated that children and asthma were risk factors associated with SRs for patients who received SCIT. Children, asthma, and LR/LLR were risk factors associated with SRs for SCIT injections (Table 6).
Systemic reactions have attracted great attention in the practice of allergen immunotherapy (AIT) because of possible anaphylaxis and severe consequences. Multi-factors, such as allergen extracts, treatment regimen, and concomitant diseases/medications, have been identified to contribute to the incidence of SRs,23 which lead to the reported rates of SRs varying considerably between studies. In recent years, several practice parameters have been published to provide principles of safe and effective administration of AIT.178 These parameters have helped physicians standardize several aspects of AIT practice and have provided guidelines to improve safety and reduce adverse effects. AIT has been applied for more than 50 years in China, while for lack of standardized allergen extracts and uniform treatment procedure, the efficacy and safety profile of SCIT were inconsistent and under controversy. A survey by Chinese otolaryngologists of trends in specific immunotherapy (SIT) for allergic rhinitis showed the majority of respondents considered SIT relatively controllable and safe, but more than half of them were still concerned about the possibility of local and systemic reactions with a clearly defined SR grading system,9 and most otolaryngologists cannot justify SCIT because of its potentially fatal systemic complications in patients with AR.10 Nowadays, dust mite allergen vaccine (Alutard SQ) is the only available standardized product in China. Our study aimed to investigate the incidence and risk factors of SRs to standardize dust mite immunotherapy in Central China.
The estimated SR rates in our study were 13.0% of patients and 1.1% of injections, similar to those of previous studies in China.1112 Consistent with the data reported by many studies, most (95.8%) of the SRs in our study occurred within 30 minutes of the injection. Furthermore, there were 2 delayed reactions occurring 3 and 8 hours later, which involved asthma symptoms. Several previous studies also reported that up to 50% of the SRs occurred after 30 minutes.1314151617 Thus, in addition to stay and observation for 30 minutes after injection recommended by guidelines,8 it is important to instruct patients how to deal with late reactions and when to see a doctor or call for medical help.
All systemic reactions in our study ranged from grade 1 (mild) to grade 3 (severe) and were relieved after routine symptomatic treatment, without occurrence of fatal reactions. This is probably because we followed the guidelines and strictly assessed patients' status before each injection, such as asthma control tests and PEF/FEV1 levels. With increasing vigilance against severe and fatal reactions during SCIT according to the guidelines, fatal reactions appear to be declining in recent years.1819 A 12-year survey from 1990 to 2001 estimated that fatal immunotherapy reactions occurred at a rate of 1 per 2.5 million injections, with an average of 3.4 deaths per year,20 whereas the latest data between 2008 and 2012 showed only 1 confirmed fatality among 23.3 million injection visits.19
The occurrence of SRs is likely to be dependent on the dose of allergen extracts. As shown in our study, most (91.7%) of the SRs occurred during injection of allergen extracts at the highest concentration (No. 4 vial), and SR rate was also much higher. Some studies suggest that there likely remains a significant risk at SRs in the maintenance phase when the maximum dose of allergen extracts is administrated.1521 Thus, carefully clinical evaluation before each administration is pivotal.
Interestingly, it was found that the incidence of SRs did not increase statistically significantly in patients with a history of LRs, although the SR rate was higher when an LR or an LLR preceded immediately during the injection. It is likely that an LR, especially LLR, can be a prodromal symptom in some patients. The available literature indicates that individual LRs do not appear to be predictive of subsequent SRs.2223 However, some individuals with a greater frequency of LR might be at greater risk of SRs,524 while further studies are needed to evaluate the effect of dose adjustment for LLRs.
Asthma has been considered a critical risk factor for the development of SRs during SCIT, and in particular uncontrolled asthma has been clearly associated with severe and fatal SRs. Thus, it is recommended that an assessment of asthma control and pulmonary function testing should be performed before each SCIT injection in all patients with asthma.1 This recommendation was strictly followed in our study. Both no asthmatic symptoms and PEF and/or FEV1>80% of the predicted value were taken as an indication for injection. In spite of this, asthmatic patients had a much higher rate of SRs than patients without asthma, and some other studies have also found the same result.112125 It is necessary to observe and follow up asthmatic patients more closely after SCIT injections.
We found that SR was more common among children than among adults, and the maintenance dose did not reach the maximum more commonly in children than in adults. There are a few studies showing a higher incidence of SR in children.1126 A possible explanation is that our study included more young children who might be greatly sensitized and vulnerable to allergies with less capacity of tolerance to allergen extracts. This difference may also reflect a higher proportion of children in subjects undergoing allergen-specific SCIT in Central China.
In our study, the incidence of SRs significantly increased in patients with a higher degree of sensitization to dust mites (3+/4+ SPT results, and/or serum sIgE levels of higher than grade 4). DaVeiga et al.27 found that the estimated odds ratios of SR were almost 6 times higher for patients with more than 33% (3 to 4+ positive) aeroallergen skin tests, with the odds ratio increased by 17% for each additional 4+ skin test. Moreover, some studies reported that part of the fatalities associated with immunotherapy had a high degree of sensitivity.28 It is suggested that we should be alert to the greater possibility of SRs in patients who are "reatly sensitized."
As can be seen from the above findings, high concentration of allergen extracts, injections with local reactions, asthma, young age, and high sensitivity may be associated with SRs during SCIT. Furthermore, multivariate logistic regression analysis showed that children, asthma, and concomitant LR/LLR are independent risk factors for SRs.
In conclusion, there is a low rate of systemic reactions to SCIT when recommendations of guidelines are well followed, and the severity of SRs are mild or moderate with good responses to symptomatic treatment. The increased incidence of SRs can be associated with patient state, such as asthma, childhood, high sensitivity, injection with concomitant local reactions, and high dosage. Strict assessment and close monitoring are significant for patients on SCIT, especially those with risk factors. Additionally, individual adjustment needs to be evaluated in further studies. Thus, SCIT should be performed with caution, and a tailored regimen may be necessary.
Figures and Tables
Fig. 1
A higher incidence of SR in injections of high concentration extracts (No. 4 vial). Both the SR rates of injection of No. 4 vials in a build-up phase (1.71%) and a maintenance phase (1.78%) were significantly higher than those of No. 3 vials (0.33%) and No. 2 vials (0.32%) (P4B-3=0.012, P4B-2=0.011, P4M-3=0.009, P4M-2=0.009). However, there was no significant difference in SR rates of injection of No. 4 vials between the build-up and maintenance phases (P=0.9). *P<0.05; **P<0.01. B, build-up phase; M, maintenance phase.
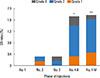
Fig. 2
Age as a risk factor for systemic reactions. Boxplot demonstrating the age distribution of patients with/without SRs. The median (25th, 75th) ages were 7 (6, 12) in patients with SR and 15 (8, 33) in those without, and the differences were statistically significant (all P<0.001). Line inside the box=median. Bottom of the box=25th; top of the box=75th percentile.  =outliers. Upper whisker=maximum observation below the upper fence (75th percentile +1.5 Q); lower whisker=minimum observation above the lower fence (25th percentile -1.5 Q); Q=quartile range.
=outliers. Upper whisker=maximum observation below the upper fence (75th percentile +1.5 Q); lower whisker=minimum observation above the lower fence (25th percentile -1.5 Q); Q=quartile range.

Table 1
Diseases and sensitization features in different age subgroups

|
Children n=121 |
Adults n=87 |
P value | |
|---|---|---|---|
| Diseases asthma (%) | 44 (36) | 24 (28) | 0.183 |
| SPT | |||
| Greatly sensitized (%) | 45 (37) | 30 (34) | 0.688 |
| Serum sIgE | |||
| Greatly sensitized (%) | 104 (86) | 52 (60) | <0.001 |
Table 2
Manifestations and severity of systemic reactions

Table 3
Administered rescue medications for systemic reactions

| AH | B | AH+B | AH+B+CS | |
|---|---|---|---|---|
| No. of reactions | 11 | 12 | 17 | 7 |
| Grade | 11 grade 1 | 12 grade 2 | 14 grade2 | 3 grade2 |
| 3 grade3* | 4 grade3* |
Table 4
SR rates in different subgroups divided according to sex, age, disease, and sensitization

Table 5
SR rates of 121 children patients in different subgroups
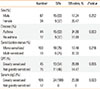
Table 6
Risk factors for SRs to SCIT analyzed by logistic regression

References
1. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127:Suppl. S1–S55.
2. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102:558–562.
3. Rank MA, Bernstein DI. Improving the safety of immunotherapy. J Allergy Clin Immunol Pract. 2014; 2:131–135.
4. Li J, Huang Y, Lin X, Zhao D, Tan G, Wu J, et al. Factors associated with allergen sensitizations in patients with asthma and/or rhinitis in China. Am J Rhinol Allergy. 2012; 26:85–91.
5. Roy SR, Sigmon JR, Olivier J, Moffitt JE, Brown DA, Marshall GD. Increased frequency of large local reactions among systemic reactors during subcutaneous allergen immunotherapy. Ann Allergy Asthma Immunol. 2007; 99:82–86.
6. Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010; 125:569–574.
7. Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. 2006; 61:Suppl 82. 1–20.
8. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3.
9. Zhou H, Tao QL, Wei JM, Xu G, Cheng L. Trends in specific immunotherapy for allergic rhinitis: a survey of Chinese ENT specialists. Allergy Asthma Immunol Res. 2014; 6:296–303.
10. Rhee CS. Current specific immunotherapy for allergic rhinitis: perspectives from otorhinolaryngologists. Allergy Asthma Immunol Res. 2014; 6:273–275.
11. Chen J, Li B, Zhao Y, Zhang Q, Wan L, Liu J, et al. A prospective multicenter study of systemic reactions in standardized specific immunotherapy for allergic rhinitis in China. Am J Rhinol Allergy. 2014; 28:e40–e44.
12. Wang H, Lin X, Hao C, Zhang C, Sun B, Zheng J, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy. 2006; 61:191–197.
13. DaVeiga SP, Caruso K, Golubski S, Lang DM. A Retrospective Survey of Systemic Reaction from Allergen Immunotherapy. J Allergy Clin Immunol. 2008; 121:suppl. S124.
14. Lin MS, Tanner E, Lynn J, Friday GA Jr. Nonfatal systemic allergic reactions induced by skin testing and immunotherapy. Ann Allergy. 1993; 71:557–562.
15. Rank MA, Oslie CL, Krogman JL, Park MA, Li JT. Allergen immunotherapy safety: characterizing systemic reactions and identifying risk factors. Allergy Asthma Proc. 2008; 29:400–405.
16. Schiappoli M, Ridolo E, Senna G, Alesina R, Antonicelli L, Asero R, et al. A prospective Italian survey on the safety of subcutaneous immunotherapy for respiratory allergy. Clin Exp Allergy. 2009; 39:1569–1574.
17. Winther L, Arnved J, Malling HJ, Nolte H, Mosbech H. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006; 36:254–260.
18. Bernstein DI, Epstein T, Murphy-Berendts K, Liss GM. Surveillance of systemic reactions to subcutaneous immunotherapy injections: year 1 outcomes of the ACAAI and AAAAI collaborative study. Ann Allergy Asthma Immunol. 2010; 104:530–535.
19. Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008-2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. 2014; 2:161–167.
20. Bernstein DI, Wanner M, Borish L, Liss GM. Immunotherapy Committee. American Academy of Allergy. Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004; 113:1129–1136.
21. Moreno C, Cuesta-Herranz J, Fernández-Távora L, Alvarez-Cuesta E. Immunotherapy Committee. Sociedad Española de Alergología e Inmunología Clínica. Immunotherapy safety: a prospective multi-centric monitoring study of biologically standardized therapeutic vaccines for allergic diseases. Clin Exp Allergy. 2004; 34:527–531.
22. Kelso JM. The rate of systemic reactions to immunotherapy injections is the same whether or not the dose is reduced after a local reaction. Ann Allergy Asthma Immunol. 2004; 92:225–227.
23. Tankersley MS, Butler KK, Butler WK, Goetz DW. Local reactions during allergen immunotherapy do not require dose adjustment. J Allergy Clin Immunol. 2000; 106:840–843.
24. Calabria CW, Stolfi A, Tankersley MS. The REPEAT study: recognizing and evaluating periodic local reactions in allergen immunotherapy and associated systemic reactions. Ann Allergy Asthma Immunol. 2011; 106:49–53.
25. Tabar AI, García BE, Rodríguez A, Olaguibel JM, Muro MD, Quirce S. A prospective safety-monitoring study of immunotherapy with biologically standardized extracts. Allergy. 1993; 48:450–453.
26. Gamboa P, González G, Jauregui I, Jorró G, Molero I, Eseverri JL, et al. A prospective and multicenter safety-monitoring study of a short up-dosing schedule of immunotherapy with a mass-units-standardized extract of mites. Allergol Immunopathol (Madr). 2004; 32:13–17.
27. DaVeiga SP, Liu X, Caruso K, Golubski S, Xu M, Lang DM. Systemic reactions associated with subcutaneous allergen immunotherapy: timing and risk assessment. Ann Allergy Asthma Immunol. 2011; 106:533–537.e2.
28. Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985-1989. J Allergy Clin Immunol. 1993; 92:6–15.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download