Abstract
Purpose
The basophil activation test (BAT) has been used to monitor venom immunotherapy (VIT) due to its high specificity. A previous study has reported a good correlation between a significant decrease in basophil activation during 5 years of VIT and clinical protection assessed by sting challenge. The following prospective study was performed to examine changes in basophil reactivity over a complete VIT period of 5 years.
Methods
BAT in a dose-response curve was studied prospectively in 10 hymenoptera venom-allergic patients over 5 years of VIT. BAT was performed at the time of diagnosis, 1 month after finishing the VIT build-up phase, and 3, 6, 12, 24, and 60 months after beginning treatment. The repeated measures ANOVA was applied to evaluate basophil activation changes throughout VIT. A cross-sectional study was also performed in 6 patients who received treatment for more than 3 years, and in another 12 patients who followed immunotherapy for at least 5 years.
Results
An early activation decrease was observed during the first 3 months of treatment, compared to pre-treatment values. This activation decrease was not maintained 6 to 18 months after treatment, but was observed again after 2 years of treatment, and maintained until the completion of the 5-year immunotherapy period. In cross-sectional analysis, the 6 patients who received treatment for 3 years, and 9 of the 12 patients who received treatment for 5 years, had negative BAT results. Three patients in this last group had positive BAT results and 2 patients had systemic reactions after field stings.
Allergy to hymenoptera venom is a potentially life-threatening disease, but long-term control is possible through venom immunotherapy (VIT). Its efficacy is high, protecting around 95% of patients allergic to Vespidae and 75%-85% of those allergic to honey bees.12 Sometimes, it is necessary to increase the conventional dose to achieve protection.3
Unfortunately, routine diagnostic tests, skin tests, and specific IgE are not feasible for evaluating the clinical protection of patients following VIT.45678 Five years after immunotherapy, more than 50% of patients have detectable specific IgE and no significant changes in cutaneous tests sensitivity.9 Although revealing studies about genetics and other biomarkers are ongoing,101112 currently the in vivo test that provides more information about individual clinical protection achieved by a particular treatment is the sting challenge test. Nevertheless, it has several drawbacks, such as risk of anaphylaxis, difficulty in obtaining some species of hymenoptera, and the fact that it requires a fair amount of time, as well as human and material resources. Indeed, the main inconvenience of this test is that it does not offer a 100% negative predictive value when only one sting is tolerated, due to the influence of the quality of the venom and several individual biological factors associated with clinical reactivity.91314 Repeated stings, however, increase the complexity and cost of patient follow-up, and are not recommended due to the elevated risk of adverse reactions.
Undoubtedly, a non-invasive test would be optimal to detect unprotected patients. The basophil activation test (BAT) has been used for the diagnosis of hypersensitivity to inhalants, latex, food, and drugs.151617 It is a very sensitive and specific method to diagnose venom allergy.1819202122 Its high specificity is due to the fact that it studies cellular response following antigen stimulation in real time. This makes it possible to monitor VIT by using serial BAT determinations, at both short-and the long term follow-up and in cross-sectional approaches.1623242526272829 In a long-term study on induction of tolerance after a complete period of at least 4.5 years of VIT monitored by using BAT,28 22 of the 23 patients treated tolerated sting challenge after finishing VIT. The clinical tolerance was correlated with an approximately 4-fold decrease in basophil responsiveness, and this drop was sustained 3 to 6 months after the sting. In contrast, in a single patient who had a positive challenge the basophil response did not change during VIT. This significant change in basophil reactivity was also assessed in other studies that compared 17 bee or wasp allergic patients who tolerated field sting after finishing VIT with 14 other reactive patients whose basophil activation did not change after completion of VIT.26 In another study, a group of 27 patients treated with bee venom IT for almost 5 years tolerated sting challenge, and BAT achieved a negative predictive value of 100%.29 In children, the decrease in basophil reactivity 4 years after VIT has been assessed in 85% of a series of 31 patients, and approximately a half of them reported tolerance to field sting.30
The following prospective study examined changes in basophil reactivity over a complete VIT period of 5 years. A cross-sectional study was also performed in 2 other groups of patients, one comprising patients treated for almost 3 years, and the other comprising those who had completed a course of 5 years of immunotherapy.
The subjects of the study were 10 patients allergic to hymenoptera venom presenting systemic reactions, 8 men and 2 women, between the ages of 14 and 76 years. Two of them were allergic to Apis mellifera, 3 to Vespula germanica, and 5 to Polistes dominulus (Table 1).
The diagnosis was established using serum specific IgE measurements (ImmunoCap, Thermo Fisher Scientific, Waltham, MA, USA) and skin prick and intradermal tests (Venom Pharmalgen, ALK-Abello, Madrid, Spain). Endpoint titration was used for intradermal tests (10-5 to 10-1 µg/mL), according to the guidelines.31
VIT was prescribed for all of them (Pharmalgen, ALK-Abello, Madrid, Spain). A 6-week build-up schedule was followed up to a maintenance dose of 100 mcg, administered monthly.
Serial BAT was performed before beginning VIT and over the following period of 4.5-5 years. Baseline BAT determination was made at the time of diagnosis (0m, basal); the next analysis was performed 1 month after finishing the VIT build-up phase (1m), immediately before receiving the first monthly maintenance dose, and subsequent measurements were taken after 3, 6, 12, 18, 24, and 60 months of treatment, named as 3m, 6m, 12m, 18m, 24m, and 60m, respectively. The samples were taken 1 month after starting the maintenance dose —which is on study— was administered. The analyses that could not performed or were not valuable at the mentioned times due to intercurrent medical processes or non-attendance of patients, were considered lost, to avoid a hypothetical bias caused by its performance 1 or more months later; as all the results corresponding to each time were analyzed in block.
Ten healthy controls without detectable venom-specific IgE or clinical histories of hymenoptera sting adverse reactions were studied.
A cross-sectional study was also performed to examine basophil activation in a group of patients who were in treatment for more than 3 years (n=6), and in another group of patients who received immunotherapy for almost 5 years (n=12) (Table 2). No baseline determinations were available for this series, as treatments began before starting the study protocol. In the first subgroup, 4 patients were allergic to Apis and 2 patients to Wasps. In the group who finished VIT, 8 patients were allergic to Apis, 2 patients to Polistes, 1 to Vespula and 1 patient to both Vespula and Polistes.
Accidental stings were registered during the follow-up period in order to correlate clinical protection with BAT results.
All the individuals involved were informed and signed the informed consent.
The method of BAT is described above.173233 The analyses were performed within 24 hours of sampling (heparinized tubes; Vacutest Kima, Arzergrande,Italy). The whole blood was distributed in aliquots and pre-incubated for 10 minutes at 37℃ with a stimulation buffer containing IL-3 (3 ng/mL, Thermo Scientific, Rockford, IL, USA). Straightaway, the samples were stimulated for 20 minutes at 37℃ in a 100-µL water bath containing the tested stimulus: positive and negative controls as well as serial dilutions of commercial venom extracts (Alk-Abello, Madrid, Spain). The extraction solvent was used as a negative control. The chemotactic peptide fMLP (N-formyl-methionyl-leucyl-phenylalanine) working solution was tested as a positive control (Sigma-Aldrich, St. Louis, MO, USA). The venom dose-response curve was tested in each BAT and included 4 final concentrations (25, 100, 500, and 1,000 ng/mL), selected as explained below. After the incubation process, the samples were left on ice for 5 minutes. A double surface staining with 10 µL of each monoclonal antibody was employed according to the following strategy: CD203c-PE (Beckman Coulter, Brea, CA, USA) for gating the basophil population and CD63-FITC (Beckman Coulter) for measuring basophil activation. After staining, the samples were incubated for 20 minutes at 4℃, light protected, lysed for 10 minutes (Optilyse C, Beckman Coulter), centrifuged at 250×g for 5 minutes and washed. After decanting the supernatant, the samples were re-suspended in 400 µL of PBS and analyzed within 1 hour using flow cytometry. The basophils were gated as CD203c-positive cells, as many as 500 events per sample, and CD63 co-expression was measured. The analysis was performed on a FC500 flow cytometer using MXP and Kaluza softwares (Beckman Coulter).
To select adequate concentrations, a wide dose-response curve, from 2.5 to 5,000 ng/mL (2.5, 5, 10, 25, 100, 500, 1,000, 2,500, and 5,000 ng/mL) was basally tested in 20 venom allergic patients complaining of systemic reactions. The criterion for positive results was the one described above for allergic patients, performing ROC-curves and using the same laboratory technique.18192023 So, it was required that after adding the venom to the sample, a minimum of 15% of basophils became activated (measured as CD63 expression), after subtracting the basal activation seen on the negative control. It was also desirable that the negative control did not exceed the 5% of basally activated basophils and that the positive control almost doubled the negative. According to this criterion, 3 of the patients were nonresponsive to the positive control and 41.66% of the series showed positive results for concentrations equal to or over 25 ng/mL; 50% over 100 ng/mL; 91.66% over 500 ng/mL; and all responsive patients over 1,000 ng/mL. The same curve was tested in 10 individual controls. No positive BAT results were found in any health controls, even for the highest concentration of 5,000 ng/mL.
Based on these results, in order to optimize the assay protocol in terms of efficiency, 4 concentrations were tested in all the analyses conducted throughout the VIT period: 25, 100, 500, and 1,000 ng/mL.
In the cross-sectional studies carried out in patients who received treatment during more than 3 years and those who completed 5 years of VIT, 2,000 and 5,000 ng/mL concentrations were also tested (dose-response curve for cross-sectional assays: 25, 100, 500,1,000, 2,000, and 5,000 ng/mL).
The statistical analysis was performed with IBM SPSS Statistics 19.00. Basophil activation was measured as the percentage of CD63+ basophils (basophil reactivity) for 4 different concentrations from the 8 tests conducted throughout the VIT period. The repeated measures ANOVA was applied to evaluate changes in basophil activation during VIT (The activation results obtained with all the concentrations tested at each time of analysis were compared in blocks to those of the other times of analysis). Mauchly's test of sphericity (The condition where the variances of the differences between all combinations of related groups are equal) indicated that the assumption of sphericity was violated, and therefore, a Greenhouse-Geisser correction was used. The Q-Q plot (quantile-quantile plot) and the Shapiro-Wilk test were used for checking normality. A P value of ≤0.05 was required for statistical significance.
With regard to basophil reactivity during VIT, an early decrease in basophil activation was observed within the first 3 months of treatment, compared to pre-treatment levels. This decrease was not maintained 6 to 18 months of treatment, but it was assessed again after 2 years of treatment and maintained throughout the remaining 3 years of VIT. The dose-response shaped (Fig. 1) according to these results was observed for all the concentrations tested and similar for all the series of patients (Fig. 2). As a result, the increase in venom concentrations was related to major basophil activation values.
Analyzing the results at each analysis time (Fig. 2), a significant decrease in basophil activation was found in 3m (P=0.046) (3 months of treatment) in comparison to pre-treatment levels. This trend of early decrease in basophil reaction was also observed in 1m, although it did not achieve significance (P=0.056). As mentioned above, this decrease was not maintained at intermediate times of treatment. Subsequently, significant differences in basophil activation levels were observed between the low activation levels assessed in 3m and the increase in basophil activation found in 6m (P=0.042) (Figs. 1 and 2). A significant decrease in basophil activation was also found after 2 years of treatment (24m), until the completion of the 5-year VIT period (60m), similar to that described for the first three months of treatment. Significant differences were observed between 18 m (when basophil activation levels did not differ significantly from pre-treatment levels) and 24m (P=0.05) (after which the activation reduction was maintained throughout the rest of VIT). The described behavior of basophil activation explains the negativization of BAT results during the treatment, mainly in the assays performed at the first months of VIT and 2 years after VIT treatment, until completing VIT (Table 3).
The ratio of tests that met the positivity criterion was also analyzed. It was not the objective of the study to evaluate the qualitative parameter of positivity, more important for diagnostic purpose than for monitoring VIT. However, at certain times of treatment, although the reduction in the CD63+ basophil percentage was not significant with regard to basal values, the activation results decreased below the positivity criterion used to discriminate healthy individuals from allergic patients. BAT negativization at the beginning of VIT is more evident for the lower concentrations tested and indicates a decrease in basophil sensitivity. For the highest concentrations the negativization was observed in the last years of treatment. So, when the results for the concentration of 100 ng/mL were analyzed separately, there were early decreases in basophil reactivation in 1m and 6 m with regard to basal values, which were not evident for 500 ng/mL at both time points (Fig. 3). Then, before beginning VIT, 62.15% of the total tests performed were positive, in contrast to 23.33% in 1m, 28% in 3m, 30% in 6m, 38.8% in 12m, 15.78% in 18m, and 21.05% in 24m. In the last time point (60m), no positive results were obtained.
Accidental stings were registered during the follow-up period in order to determine if there is a correlation between activation rates and clinical protection. Two patients were stung during the build-up phase (in the fifth and sixth weeks of the protocol), 2 patients at the 12th month of treatment and one last at the 15th, respectively (Table 1). None of them reported systemic reactions, and no remarkable changes in basophil activation were found in comparison to subsequent analysis results.
In the group of 6 patients who completed at least 3 years of VIT, all the patients presented negative BAT results, and 2 patients reported good tolerance to spontaneous stings (Table 2).
In the subgroup of 12 patients who completed 5 years of VIT, 9 patients presented negative BAT results throughout the dose-response curve, including high concentrations, 4 of whom reported good clinical tolerance to spontaneous stings (Table 2). Three patients presented positive results (1 patient for Apis, 1 patient for Polistes, and 1 poli-sensitized patient for both Vespula and Polistes), and the last 2 reported systemic reactions to field stings. In these 2 cases, activation was over 15% of the basophil population for as low a concentration as 25 ng/mg, reaching levels of 90% for 1,000 ng/mL. The clinical tolerance of the patient allergic to Apis who was not stung again is unknown.
Although similar studies concerning VIT monitoring by using BAT have been published, in this work the longitudinal controls throughout a complete 5 years period of treatment were performed more frequently than those previously reported, and a wider range of concentrations was tested. The close monitoring carried out in this study can in part clarify discordant results among several studies in terms of changes in basophil activation during VIT. The results of the intermediate-and long-term cross-sectional studies support the results obtained in the prospective analysis.
With regard to the treatment length necessary to observe significant changes in basophil activation, according to previous reports, we found no significant decrease in basophil activation in the analysis performed 623 and 12 months after the initiation of VIT;28 however, it was significantly observed 3 months after starting the maintenance dose, and nearly significant in the first month. Actually, the studies by Ebo et al.24 showed an early significant decrease in basophil activation immediately after a 5-day semi-rush schedule, but only for submaximal concentrations (10 ng/mL), which represents a 10-fold decrease in basophil sensitivity. In our and other series after 6 months of treatment, a significant decrease was observed for 10 and 100 ng/mL,2430 but not for a maximal concentration of 10,000 ng/mL.24 In fact, in our series, the early decrease in basophil activation was more evident when analyzed at a concentration of 100 ng/mL, but not for the highest concentrations tested (Fig. 3). Then, this drop in basophil activation in the first year can be overlooked if submaximal concentrations are not tested.
On the other hand, in order to adequately interpret basophil activation results obtained in each time of analysis, it must be taken into account that basophil activation values can be influenced by each patient's basophil sensitivity threshold. As seen in the prospective series and in the group of 20 patients where the initial dose-response curve was performed, several patients showed high responsiveness rates for low concentrations, in comparison to those who needed more quantity of allergen to become activated. To avoid such interference with the evaluation of our prospective series, the results of all the concentrations tested each time were analyzed in blocks by repeated measures ANOVA which allows the evaluation of the global evolution of basophil response during the VIT and helps overcome this problem. Nevertheless, other analysis methods, such as the CD-sens, which was not performed in this study, can have also been adequate in this study.
In our study, early changes in basophil activation were not maintained between 6 and 18 months of treatment; however, a significant decrease in basophil activation was observed once again after 2 years of treatment (or 1 and a half years for several patients, in the person-to-person analysis). In the cross-sectional controls performed in the third and fifth years of treatment, results were also comparable. Ebo et al.24 found similar results in cross-sectional controls of patients treated for 3 years, which shows significantly lower activation levels than the pre-treatment levels in other series.
With regard to the clinical relevance of basophil activation levels, a significant decrease in basophil reactivity in patients who tolerated sting challenge,262829 and field stings30 have been associated with a good predictive value for clinical protection. This correlation could not be assessed in our series because few field stings have been reported by our patients (Tables 1 and 2). In fact, VIT monitoring by using BAT was considered to obtain information about the clinical evolution of patients during VIT while dispensing the challenge. This fact limits conclusions about clinical protection presumably achieved by the patients in whom the decrease in basophil activation was found to be statistically significant and whose basophil behavior was similar to those of previously reports for patients who tolerated re-stings.26282930 In the group of patients treated for almost 5 years, BAT was negative in 75% of patients, and those who suffered from systemic reactions after completing VIT showed positive BAT results at the lowest concentration tested. It would not be practicable to monitor VIT so closely using challenge testing. Further studies in larger populations are needed to confirm our results.
Currently, it is not possible to fully explain the reproducible activation increase in the intermediate-term analysis (6 to 18 months of treatment). A similar response has been described in a previous study on sublingual immunotherapy (SLIT) with latex, where no significant activation changes in basophil activation were observed after 1 year of treatment, but were found after 2 years.34 Again, no early decrease in basophil activation was observed after 4 months of grass SLIT3536 and 6 months of peach SLIT;37 in fact, an increase was observed in the last study.
A plausible pathophysiological explanation for these results could be immunological changes observed in the early phases of immunotherapy with foods and other allergens, such as the allergen-specific IgE increase,38394041 possibly resulting in a reduction in the basophil responsiveness threshold or the antigen-specific CD4+ T-cell proliferation induced by basophils.4243 Indeed, previous studies have shown that CD63 expression does not always reflect mediator release,44 as assessed by in vitro studies and good clinical tolerance to stings. This fact probably reflects an initial desensitization of basophils, where signalling cellular activation still works, but degranulation does not take place. We are currently conducting assays where basophil activation and histamine release are measured simultaneously in order to clarify the significance of basophil responsiveness in these intermediated phases of VIT (data not shown). Thus, far from concluding that BAT is not a useful method to monitor specific immunotherapy,3536 the reproducible assessment of these immunological changes, found in all the patients prospectively examined (Fig. 2), is probably showing the initial stages of a long-lasting protective effect of this treatment. Thus, BAT results might be carefully interpreted after taking into account the length of the treatment and the venom concentration tested at each analysis time, which should be adequate to such length. Different responses between individual patients could indicate a suboptimal response to immunotherapy, and therefore, early therapeutic modifications can be made, such as increasing the maintenance dose and the therapeutic period, or adding coadjutant drugs, such as omalizumab or epinephrine as a rescue.
Considering the best way to monitor the individual response to VIT, it must be taken into account that basophil threshold sensitivity varies, not only through the time, but also among patients. Hence, a dose-response curve is more sensitive to evaluate changes in basophil activation throughout the treatment period. As mentioned above, 41.66% of the 20 patients, in whom the complete dose-response curve (2.5 to 5,000 ng/mL) was basally created, showed positive results at a concentration of 25 ng/mL. Therefore, testing at lower concentrations could be helpful in monitoring more sensitive patients, especially in the early phases of VIT. A 4-concentration dose-response curve is proposed to be tested at the first 3 years of VIT (25, 100, 500, and 1,000 ng/mL). On a long-term basis, after 3 years of treatment, testing at higher venom concentrations (2,000 and 5,000 ng/mL) is advisable to evaluate the risk of anaphylaxis. As Zitnik et al.30 described, patients with worse tolerance in the build-up phase showed a greater value of 0.1/1 index for basophil activation.
Finally, although more longitudinal comprehensive studies would be of great interest, the activation results in the intermediate-and long-term changes in basophil reactivity suggest that BAT could help monitor VIT safely and effectively. BAT can be performed at any time as needed in order to detect nonresponsive patients and to determine the need to take additional therapeutic measures.
Figures and Tables
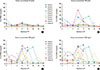 | Fig. 2Changes in basophil activation at the 8 time points of analysis are shown separately for 4 concentrations tested: 25 (A), 100 (B), 500 (C), and 1,000 ng/mL (D). |
 | Fig. 3Changes in basophil activation at 1 and 6 months after beginning treatment at concentrations of 100 and 500 ng/mL. The proportion of basal activation results is represented in the upper part of the box-plot, and the proportion after the treatment in the lower. (A) changes at 1 month of treatment at 100 ng/mL; (B) changes at 1 month of treatment at 500 ng/mL; (C) changes at 6 months of treatment at 100 ng/mL; (D) changes at 6 months of treatment at 500 ng/mL. |
Table 1
A series of patients followed up prospectively

Table 2
Cross-sectional series

ACKNOWLEDGMENTS
This study is financially supported by Fundesalud (the Public Health Service of Extremadura Foundation to promote the investigation).
Aknowledgements to Stallergenes Iberica laboratories for the writing assistance.
Aknowledgements to Dr. Rosa Miriam Blanco Pérez for her contribution to patient follow-up.
References
1. Müller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol. 1992; 89:529–535.
2. Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005; 115:439–447.
3. Ruëff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. 2001; 108:1027–1032.
4. Keating MU, Kagey-Sobotka A, Hamilton RG, Yunginger JW. Clinical and immunologic follow-up of patients who stop venom immunotherapy. J Allergy Clin Immunol. 1991; 88:339–348.
5. van Halteren HK, van der Linden PW, Burgers JA, Bartelink AK. Discontinuation of yellow jacket venom immunotherapy: follow-up of 75 patients by means of deliberate sting challenge. J Allergy Clin Immunol. 1997; 100:767–770.
6. Graft DF. Discontinuing venom immunotherapy. J Allergy Clin Immunol. 1997; 99:271.
7. Golden DB, Kwiterovich KA, Kagey-Sobotka A, Lichtenstein LM. Discontinuing venom immunotherapy: extended observations. J Allergy Clin Immunol. 1998; 101:298–305.
8. Lerch E, Müller UR. Long-term protection after stopping venom immunotherapy: results of re-stings in 200 patients. J Allergy Clin Immunol. 1998; 101:606–612.
9. Ruëff F, Przybilla B, Müller U, Mosbech H. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996; 51:216–225.
10. Akdis CA, Blesken T, Akdis M, Wüthrich B. BlaserK. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998; 102:98–106.
11. Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010; 65:1558–1565.
12. Konno S, Golden DB, Schroeder J, Hamilton RG, Lichtenstein LM, Huang SK. Increased expression of osteopontin is associated with long-term bee venom immunotherapy. J Allergy Clin Immunol. 2005; 115:1063–1067.
13. Dubois AE, de Monchy JG. Sting challenge outcome as a selection criterion for venom immunotherapy. J Allergy Clin Immunol. 1997; 100:144–146.
14. Bilò MB, Brianzoni MF, Garritani MS, Antonicelli L, Farabollini B, Bonifazi F. The sting challenge test in Hymenoptera venom allergy: pros and cons. Eur Ann Allergy Clin Immunol. 2003; 35:377–381.
15. Sainte-Laudy J, Vallon C, Guérin JC. Analysis of membrane expression of the CD63 human basophil activation marker. Applications to allergologic diagnosis. Allerg Immunol (Paris). 1994; 26:211–214.
16. Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008; 74:201–210.
17. Rodríguez-Trabado A, Cámara-Hijón C, Ramos-Cantariño A, Porcel-Carreño SL, Jiménez-Timón S, Pereira-Navarro G, et al. Basophil activation test for the in vitro diagnosis of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Proc. 2008; 29:241–249.
18. Sturm GJ, Böhm E, Trummer M, Weiglhofer I, Heinemann A, Aberer W. The CD63 basophil activation test in Hymenoptera venom allergy: a prospective study. Allergy. 2004; 59:1110–1117.
19. Eberlein-König B, Schmidt-Leidescher C, Rakoski J, Behrendt H, Ring J. In vitro basophil activation using CD63 expression in patients with bee and wasp venom allergy. J Investig Allergol Clin Immunol. 2006; 16:5–10.
20. Korosec P, Erzen R, Silar M, Bajrovic N, Kopac P, Kosnik M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin Exp Allergy. 2009; 39:1730–1737.
21. Eberlein-König B, Rakoski J, Behrendt H, Ring J. Use of CD63 expression as marker of in vitro basophil activation in identifying the culprit in insect venom allergy. J Investig Allergol Clin Immunol. 2004; 14:10–16.
22. Ebo DG, Hagendorens MM, Bridts CH, De Clerck LS, Stevens WJ. Hymenoptera venom allergy: taking the sting out of difficult cases. J Investig Allergol Clin Immunol. 2007; 17:357–360.
23. Erdmann SM, Sachs B, Kwiecien R, Moll-Slodowy S, Sauer I, Merk HF. The basophil activation test in wasp venom allergy: sensitivity, specificity and monitoring specific immunotherapy. Allergy. 2004; 59:1102–1109.
24. Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck LS, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom. 2007; 72:196–203.
25. Mikkelsen S, Bibby BM, Dolberg MK, Dahl R, Hoffmann HJ. Basophil sensitivity through CD63 or CD203c is a functional measure for specific immunotherapy. Clin Mol Allergy. 2010; 8:2.
26. Peternelj A, Silar M, Erzen R, Kosnik M, Korosec P. Basophil sensitivity in patients not responding to venom immunotherapy. Int Arch Allergy Immunol. 2008; 146:248–254.
27. Čelesnik N, Vesel T, Rijavec M, Šilar M, Eržen R, Košnik M, et al. Short-term venom immunotherapy induces desensitization of FcεRI-mediated basophil response. Allergy. 2012; 67:1594–1600.
28. Eržen R, Košnik M, Silar M, Korošec P. Basophil response and the induction of a tolerance in venom immunotherapy: a long-term sting challenge study. Allergy. 2012; 67:822–830.
29. Hausmann O, Diaz C, Schneider M, Weber J, Pecaric-Petkovic T, Helbling A. Correlation of sting challenge outcome and change in EC50 in basophil activation test (BAT) in bee venom allergic patients after 2-5 years of venom immunotherapy. Allergy. 2014; 69:Suppl 99. 111.
30. Žitnik SE, Vesel T, Avčin T, Šilar M, Košnik M, Korošec P. Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr Allergy Immunol. 2012; 23:166–172.
31. Position paper: Allergen standardization and skin tests. The European Academy of Allergology and Clinical Immunology. Allergy. 1993; 48:48–82.
32. Rodríguez Trabado A, Fernández Pereira LM, Romero-Chala S, García-Trujillo JA, Cámara Hijón C. Evaluation of latex subclinical sensitization by way of the basophil activation test and specific IgE to latex recombinant allergens. Allergol Int. 2013; 62:385–387.
33. Rodríguez Trabado A, Cámara Hijón C, Porcel Carreño SL, Rodríguez Martín E, Fletes Peral C, Pereira Navarro G, et al. Anaphylaxis caused by cloxacillin: diagnosis with seriated analysis by way of basophil activation test. Allergy Asthma Proc. 2006; 27:269–272.
34. Gastaminza G, Algorta J, Uriel O, Audicana MT, Fernandez E, Sanz ML, et al. Randomized, double-blind, placebo-controlled clinical trial of sublingual immunotherapy in natural rubber latex allergic patients. Trials. 2011; 12:191.
35. Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Devillier P, Montagut A, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009; 124:471–477.
36. Van Overtvelt L, Baron-Bodo V, Horiot S, Moussu H, Ricarte C, Horak F, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011; 66:1530–1537.
37. Garrido-Fernández S, García BE, Sanz ML, Echechipía S, Lizaso MT, Tabar AI. Are basophil activation and sulphidoleukotriene determination useful tests for monitoring patients with peach allergy receiving sublingual immunotherapy with a Pru p 3-enriched peach extract? J Investig Allergol Clin Immunol. 2014; 24:106–113.
38. Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015; 7:221–229.
39. Akkoc T, Akdis M, Akdis CA. Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res. 2011; 3:11–20.
40. James LK, Durham SR. Update on mechanisms of allergen injection immunotherapy. Clin Exp Allergy. 2008; 38:1074–1088.
41. Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009; 10:697–705.
42. Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009; 10:713–720.
43. Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009; 10:706–712.
44. Jutel M, Müller UR, Fricker M, Rihs S, Pichler WJ, Dahinden C. Influence of bee venom immunotherapy on degranulation and leukotriene generation in human blood basophils. Clin Exp Allergy. 1996; 26:1112–1118.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download