Abstract
Purpose
Comprehensive evaluation of anaphylaxis in China is currently lacking. In this study, we characterized the clinical profiles, anaphylactic triggers, and emergency treatment in pediatric and adult patients.
Methods
Outpatients diagnosed with "anaphylaxis" or "severe allergic reactions" in the Department of Allergy, Peking Union Medical College Hospital from January 1, 2000 to June 30, 2014 were analyzed retrospectively.
Results
A total of 1,952 episodes of anaphylaxis in 907 patients were analyzed (78% were adults and 22% were children). Foods are the most common cause (77%), followed by idiopathic etiologies (15%), medications (7%) and insects (0.6%). In food-induced anaphylaxis, 62% (13/21) of anaphylaxis in infants and young children (0-3 years of age) were triggered by milk, 59% (36/61) of anaphylaxis in children (4-9 years of age) were triggered by fruits/vegetables, while wheat was the cause of anaphylaxis in 20% (56/282) of teenagers (10-17 years of age) and 42% (429/1,016) in adults (18-50 years of age). Mugwort pollen sensitization was common in patients with anaphylaxis induced by spices, fruits/vegetables, legume/peanuts, and tree nuts/seeds, with the prevalence rates of 75%, 67%, 61%, and 51%, respectively. Thirty-six percent of drug-induced anaphylaxis was attributed to traditional Chinese Medicine. For patients receiving emergency care, only 25% of patients received epinephrine.
Conclusions
The present study showed that anaphylaxis appeared to occur more often in adults than in infants and children, which were in contrast to those found in other countries. In particular, wheat allergens played a prominent role in triggering food-induced anaphylaxis, followed by fruits/vegetables. Traditional Chinese medicine was a cause of drug-induced anaphylaxis. Furthermore, exercise was the most common factor aggravating anaphylaxis. Education regarding the more aggressive use of epinephrine in the emergency setting is clearly needed.
Anaphylaxis is a life-threatening, severe systemic allergic reaction of rapid onset.1 Its incidence appears to be rising in some Western countries.2 The symptoms of anaphylaxis are multi-systemic, involving the skin, and respiratory, gastrointestinal, and cardiovascular systems.1 Triggers of anaphylaxis may show different patterns due to differences in living environment and genetic factors as well as patient selection (e.g., outpatients vs inpatients). Food is the most common trigger, especially in children.34 Studies in the US and Europe have shown that nuts, peanuts, fish, and shellfish are common food allergens,456 whereas in some Asian countries, buckwheat and wheat are more common food triggers.78 A number of studies conducted in the US and European countries have summarized the clinical characteristics of anaphylaxis.345910 A few small studies conducted in Asia have also been published, e.g., South Korea8 and Hong Kong.11 To better understand the features of anaphylaxis in Chinese patients, we analyzed 907 anaphylactic cases retrospectively between 2000 and 2014 using the criteria for anaphylaxis developed by the National Institute of Allergy and Infectious Diseases (NIAID) in 2006.12 In this study, we characterized the clinical profiles, anaphylactic triggers, and emergency treatment in pediatric and adults patients.
Clinical data from patients diagnosed with 'anaphylaxis' or 'severe allergic reactions' from January 1, 2000 to June 30, 2014 was collected and analyzed by 3 allergists who specialized in anaphylaxis in the Department of Allergy, Peking Union Medical College Hospital. All anaphylaxis patients who were referred to our department experienced at least 1 anaphylactic reaction, and most of them had received emergency treatment in local hospitals when reactions occurred. They came to our hospital for confirming the triggers of their severe episodes. A total of 907 patients that met current criteria were included in this study. The study was approved by the Ethics Committee of Peking Union Medical College Hospital. All patients came from 31 provinces in China. Ninety-five percent were from northern China.
Assessment of the outpatients with anaphylaxis was based on the NIAID 2006 criteria12 with slight modification. Anaphylaxis is most likely when any one of the following 3 criteria is fulfilled:
(1) 2 or more of the following that occur rapidly after exposure to a likely allergen (e.g., foods, drugs, and insects) for that patient, (i) involvement of the skin-mucosal tissue (e.g., generalized hives, itch-flush, and swollen lips-tongue-uvula), (ii) respiratory compromise (e.g., dyspnea, wheeze-bronchospasm, stridor, reduced peak expiratory flow rate, and hypoxemia), (iii) reduced blood pressure (BP) or associated symptoms (e.g., hypotonia, collapse, syncope, and incontinence), and (iv) persistent gastrointestinal symptoms (e.g., crampy abdominal pain, and vomiting);
(2) experiencing similar symptoms as above (skin-mucosal tissue symptoms, and respiratory compromise/reduced BP or associated symptoms) after exposure to the same allergen;
(3) patients have been diagnosed "anaphylaxis" by physicians, and receive treatment in a hospital emergency when severe reactions occur.
The severity was stratified into mild, moderate, or severe anaphylaxis during the chart review as previously described,3 and the patient population was divided into 2 groups according to the following definition:
(1) Mild to moderate anaphylaxis: Patients with cutaneous and angioedema symptoms with additional respiratory, and gastrointestinal features. The additional features included a history of shortness of breath or dyspnea, wheeze, hoarseness, and nausea or vomiting.
(2) Severe or life-threatening anaphylaxis: Patients with any of the findings listed for mild to moderate anaphylaxis and also with potential life-threatening symptoms or signs. These included 1 or more of the following: history of loss of consciousness, syncope, or dizziness, or light-headedness at any time; systolic BP <90 mmHg; Cardiovascular system collapse and/or neurologic dysfunction from hypoperfusion, or hypoxia. Also included were patients with 1 or more of the following: history of shortness of breath, wheeze, hoarseness or bronchospasm plus any 1 or more of stridor, cyanosis, or a respiratory rate ≥25/min.
Determination of food as the trigger for anaphylaxis was based on a clear history of anaphylaxis onset within hours of ingesting food, serum specific IgE (sIgE) testing (Phadia250 Detection System, ImmunoCAP, Phadia AB, Sweden) and/or skin prick testing. Serum sIgE levels >0.35 kU/L were considered positive, skin prick testing was performed as previously described.13 A diagnosis of anaphylaxis caused by insect bites or drugs was made based on a convincing history. If the medical history did not suggest a clear trigger and all allergen tests were negative, the event was diagnosed as idiopathic anaphylaxis. Due to the potential risks of triggering anaphylaxis with oral food or drug challenges, no such testing was performed.
Demographic data, clinical features, possible triggers, specific IgE of food triggers, and treatment before hospital visits or in emergency departments were characterized.
SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables are expressed as the mean±standard deviation. Comparisons among groups were performed using ANOVA. Categorical variables are expressed as a percentage or ratio. Comparison between groups was performed using the chi-square and Fisher tests. A P value of <0.05 was considered statistically significant.
Forty-five percent (412/907) of the patients were male. The average age of first anaphylactic reaction was 31±14 years (range, 5 months-75 years). The patients were divided into 5 age groups according to the age at onset of anaphylaxis (Table 1); 68% (1,333/1,952) of the first anaphylaxis occurred at the 18-50 year age range.
The triggers for the 1,952 anaphylactic reactions are shown in Table 1; 85% of the triggers could be determined, which included foods (77%), drugs (7%) and insects (0.6%). The triggers could not be determined for the remaining 15% of all reactions.
Among 1,501 food-related anaphylactic reactions, 1,239 involved a specific food, and 262 involved unclear or mixed foods (Table 1). The most common food allergen was wheat, causing 37% of food-induced anaphylaxis. Twenty percent of food-related anaphylactic reactions were caused by fruits/vegetables, and the most common fruit was peach. Only 5% of the food-induced anaphylactic reactions were caused by tree nuts/seeds, and the most common nut was cashew. There was 1 fatal anaphylaxis induced by cashew, and the patient was male, at 75 years of age. Legume/peanut triggered 7% of food reactions. Eighty-five percent of the patients with food-induced anaphylaxis were tested for sIgE. Table 2 shows the mean levels of sIgE to common food triggers.
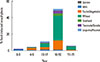
Fig. 1 shows different patterns of food triggers stratified in 5 age groups. Milk was the key trigger of anaphylaxis in the infants (62%,12/21) and young children (0-3 years; 59%, 36/61), In children (4-9 years) with anaphylaxis, 59% (36/61) episodes were triggered by fruits/vegetable, and in the teenager (10-17 years), 20% (56/282) of the anaphylactic reactions were trigged by wheat. Wheat was the leading cause of the anaphylactic reactions in adults, 42% (429/1,016) anaphylactic reactions were triggered by wheat.
Anaphylaxis induced by China-specific food included consumption of chrysanthemum tea in 6 episodes, bullfrogs in 4 episodes, silkworm chrysalis in 1 episode, locusts in 2 episodes, and cicada in 2 episodes.
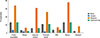
Mugwort pollen sensitization is common in patients with anaphylaxis induced by fruits/vegetables, legume/peanuts, tree nuts/seeds, and spices, with the prevalence rate of 67%, 61%, 51%, and 75%, respectively (Fig. 2). Among 24 peach-related anaphylaxis cases, 17 (71%) were allergic to mugwort.
Seven percent (143/1,952) of the anaphylactic reactions were induced by drugs. Herbs (37% of the culprit drugs) were the most common drug trigger, in which Qingkailing (14 anaphylactic reactions) was the leading cause. Antibiotics were the second most frequent drug trigger, causing 24% of drug-induced anaphylactic reactions, and 36% of these antibiotic reactions were induced by penicillin. Sixteen percent of drug-induced reactions were triggered by antipyretics and analgesics (Table 1).
We reviewed 1,952 anaphylactic reactions. Of these anaphylactic reactions, 906 were severe, potentially life-threatening anaphylaxis, with the mean onset age being 34 years, which was significantly different from the mean onset age of 27 years for 986 mild-to-moderate anaphylaxes (P<0.001). Fifty-seven percent of the severe episodes in food anaphylaxis were triggered by wheat, whereas fruits and vegetables tended to trigger mild/moderate reactions (P<0.001) (Table 3).
In total, 1,952 episodes in 907 patients with anaphylaxis were recorded in charts, wherein 324 (36%) patients were seen after their first episode by physicians and the remaining 593 patients (64%) experienced more than 1 previous episode before clinic visit. In total, the 907 patients had an average of 2.4±2.2 (range 1-40) anaphylaxis episodes, and 17 patients experienced more than 10 previous episodes. Table 4 shows characteristics of the 17 patients with more than 10 anaphylactic reactions before their visits.
Among the 1,952 anaphylactic reactions, 39% (761/1,952) occurred during exercise, 3.7% after orally taking aspirin, 1.4% occurred after alcohol consumption, 1.1% after taking non-steroidal anti-inflammatory drugs (NSAIDs) other than aspirin. Exercise involved in episodes triggered by foods (662/761, 87%) as a co-factor, and among these food- and exercise-dependent anaphylactic episodes, wheat was the leading food trigger (397/662, 60%).
Table 5 summarizes the treatment of the 1,467 anaphylactic reactions. No treatment records were available for 485 anaphylactic reactions. Among the 1,467 anaphylactic reactions with medical records, 11% resolved spontaneously, and 10% were treated at home; and 87% of those home-treated reactions received antihistamines. Seventy-nine percent of the anaphylactic reactions were treated in the emergency department. No emergency treatment records were available for 45% (521/1,161) of these reactions. Among the 640 with emergency treatment records, 72% were treated with corticosteroids and only 25% were treated with epinephrine; only 34% of the life-threatening reactions were treated with epinephrine.
The most commonly involved areas were the skin, subcutaneous tissues, and mucosa (95%). Skin symptoms were most common (78.2%), followed by respiratory tract symptoms (64.2%). Six reactions were associated with apnea. Of the 1,467 anaphylactic reactions, 56.8% had associated cardiovascular system involvement and 38.7% had gastrointestinal symptoms. Only 0.5% of the anaphylactic reactions presented as a biphasic reaction. The most common first symptom was skin (75%), followed by respiratory (16%), and gastrointestinal symptoms (7%).
This is the largest investigation concerning pediatric and adult patients with anaphylaxis in a single allergic clinic in China. Most of the prevalence data published previously regarding anaphylaxis came mainly from the emergency patients and inpatients in general and children hospitals.3458910111415 This study involved outpatients in our clinic, the biggest allergy center in China, which serves patients with allergic diseases in both adults and children from all over China.
The results of our investigation showed that the prevalence of anaphylaxis was higher in adults than in children, with the age group (18-50 years) being the most prevalent (68%). As a corollary, a hospital-based epidemiologic study from Korea8 showed that there was only 1 case (0.7%) in the children group (0-9 years) among 138 anaphylaxis patients, whereas the adult group (20-49 years) accounted for 54%. Also, anaphylaxis in children with low prevalence (2.5 per 100,000 children) was also observed in a Singapore hospital-based study. This significant adult dominance in the current study and some Asian countries like South Korea were significantly different from those of Western countries1617 which showed that the incidence of anaphylaxis was highest among children and teenagers. In fact, we have conducted a population-based epidemiological study of 9 different allergic diseases, including anaphylaxis, throughout China in 2009, this epidemiological study (based on more than 130,000 general populations from 19 provinces in China) is expected to provide more accurate information on anaphylaxis in China. However, due to the small number of cases assessed by that epidemiological investigation, it would be difficult to show general characteristics of anaphylaxis in Chinese patients, so we conducted the present study for the purpose of investigating characteristic anaphylaxis in China.
Although there is no accurate data published, allergists and pediatricians in China perceived the prevalence of anaphylaxis, especially pediatric anaphylaxis to be lower than Western countries. There are numerous published studies on anaphylaxis in Chinese journals, and we searched some Chinese citation databases and found that approximately 129 anaphylaxis studies (including case reports and clinical studies) published in 2014; among them, there were only 4 studies regarding pediatric anaphylaxis, which partially reflected that the prevalence of anaphylaxis is lower in the pediatric population than in the adult population. This is similar to the prevalence rates of food allergy and other allergic diseases in Asian children, which is much lower than those in developed countries and shows a different profile. With the development of industrialization and modernization, it is suspected that allergic diseases, including anaphylaxis, will show a similarly increasing trend and prevalence as those noted in the developed countries in the past.
In our study, the most common trigger for anaphylaxis was food (77%), which was similar to the results of several other reports,4569181920 while 15.6% of the anaphylactic reactions in our patients had unknown causes, which was on a par with the literature reporting 0.2%-15% of anaphylactic reactions with unknown triggers.1 Causes of anaphylaxis vary rather profoundly based on the geographical location and subject selection in previous studies. A study of patients from an outpatient allergy clinic in Central Europe21 showed that insect bites were the most common cause of anaphylaxis, while in South Korea, emergency patients had drugs as the most common cause.8 In comparison to studies from other countries, we observed unique patterns of food-induced anaphylaxis for Chinese patients. Wheat was the most common food to trigger anaphylaxis (37% of the food triggered episodes). To our knowledge, such a high proportion has not yet been reported in Western countries, which may be related to the lack of "gluten-free" products in China, and genetic factors may also contribute to the difference. Cai et al,22 suggested that the IL-4-C590T variant is associated with the susceptibility of wheat-dependent exercise-induced anaphylaxis in a Chinese population, although this awaits further confirmation.
Fruits and vegetables were the second most common food triggers (20% of the food-induced anaphylactic reactions). The proportion of anaphylaxis induced by fruits and vegetables has been reported to vary from 9% to 20%.562025
In contrast to North American countries where peanut was an important trigger, only 1.2% of the food-induced anaphylactic reactions in our study population were triggered by peanut. Similar findings have been reported from several Asian countries, including South Korea826 and Singapore.27
Diets in different parts of China vary widely, and foods unique to an area have been associated with the occurrence of anaphylaxis. For example, fried insects (silkworm chrysalis, locust, and cicada), chrysanthemum tea, edible potherb, bullfrogs, and turtles are considered delicacies in some geographical areas and may trigger anaphylaxis. Similarly, "bird's nest," a local delicacy in Singapore, was reported to induce anaphylaxis.27
The nature of the food allergen-induced anaphylaxis varied among different age groups. Milk was the most common food trigger in infants (0-3 years of age). Fruits were the most common food trigger in children (4-9 years of age). Wheat was the leading food trigger in teenagers (10-17 years of age) and adults (≥18 years of age). This pattern is different from other countries and regions. The most common food allergen in children from the US,25 Canada,4 and European countries920 is peanut. In adult population in the U.S.5 and Hong Kong,11 shellfish is the most common food trigger. An Italian group20 found that fruits and vegetables are the most common food allergen causing anaphylaxis in adults. Differences in food allergens are related not only to local diets, environment, and genetic factors, but also to differences in patient source and study methods.
The current study showed that mugwort sensitization was common in patients with specific food-induced anaphylaxis, especially in fruits/vegetables (67%) and spices (75%). Mugwort is the most important allergenic pollen allergen in late summer and autumn in China.28 A comprehensive review also revealed that common mugwort pollen-food syndromes include mugwort-spice syndrome and mugwort–peach association.29 We found that in patients with peach-related anaphylaxis, 71% were also allergic to mugwort pollen. Ma et al.30 recently reported that mugwort pollen sensitization was more frequently seen in symptomatic patients to peach compared to those noted in asymptomatic ones.
The proportion of drug-induced anaphylaxis was relatively low, with only 9% in our study population. This may be related to the fact that the patients included in this study were recruited from the outpatients in Allergy clinic, because the studies based on emergency department and hospitalized patients found that the proportion of drug-induced anaphylaxis was 26.9%-53%.91415 It is worth noting that in the present study, traditional Chinese medicine was a major drug allergen, while other studies have reported that antibiotics and NSAIDs were the leading drug allergens.69111415 The large number of anaphylactic reactions attributed to traditional Chinese medicine may be related to the fact that Chinese herbs are commonly used in primary Chinese hospitals. A total of 13 herbs were found to induce anaphylaxis in the current study. The most common triggers were injections of Qingkailing, Shuanghuanglian, and Houttuynia. These findings are consistent with those of Cao et al.31 who reported that 288 anaphylaxis cases were due to herb injections, among which 13 were fatal. In June 2006, the China Food and Drug Administration issued a temporary emergency suspension on the use of Houttuynia injection due to many related cases of anaphylaxis. Formulation of herb injections is relatively complex, and mechanisms underlying anaphylaxis triggered by herbs are currently unclear.
Only 0.6% of the anaphylactic reactions are triggered by insect bites, which are notably lower than those reported in the literature.46891418 A study from Central Europe21 found that 50% of the anaphylactic reactions were caused by insect bites, which are the most common cause of anaphylaxis. A study of children and teenagers in Germany18 found that bee venom (24%) was the second most common cause of anaphylaxis. A possible reason for this is that in China, patients with insect-induced anaphylaxis are preferentially referred to emergency department rather than to allergy clinics for treatment.
Seventy percent of our patients with anaphylaxis were treated with glucocorticoids, and epinephrine was used in only 26% of cases. Only 32% of severe cases received epinephrine. A relatively lower percentage (7.4%) was reported in Korean adults with anaphylaxis.32 By contrast, a study conducted in the US3 reported that 79% of the anaphylaxis patients receive epinephrine. Our findings revealed that many Chinese doctors lack a full understanding of anaphylaxis. Training and education should be provided to doctors, patients, and their families, so that they recognize the importance of epinephrine in anaphylaxis treatment. In our study, 11% of the anaphylactic reactions resolved spontaneously, including 9% of severe cases. A study of anaphylaxis in German children19 also found that 8% of severe cases resolved spontaneously. This suggests that anaphylaxis may be self-limiting; however, this does not mean the treatment of anaphylaxis should be delayed.
The present study suggested that wheat was a potential risk factor for life-threatening and recurrent anaphylaxis. As a corrolary, Mullins et al.,33 suggested that the highest risk of recurrence was associated with sensitivity to wheat. In the current study, wheat was found as a trigger in 10 (12%) of the anaphylaxis patients. Wheat allergy, especially wheat-induced anaphylaxis, is difficult to diagnose and currently clinically underestimated in China. Some patients were referred to our clinics with previous diagnosis with "idiopathic anaphylaxis." Jia et al.24 reported the first 15 Chinese cases of wheat-induced exercise-induced anaphylaxis in 2009. Complete abstinence from wheat products may be required in such cases. Accidental ingestion of wheat products or wheat allergens hidden in other food may induce recurrent anaphylaxis.
Exercise was the most common factor for aggravating anaphylaxis in our study. Forty percent of the anaphylactic reactions occurred during exercise. This finding is consistent with that of a previous study on anaphylaxis in children and teenagers in Europe.18 Seventy-four percent of the wheat-induced anaphylactic reactions were found to occur during exercise. A previous study from Japan found that wheat is the main food allergen in food-dependent exercise-induced anaphylaxis.34
The strengths of this study are that the subjects were recruited from an allergy clinic compared to evaluations of emergency department patients and inpatients, and that physicians in the allergy department could more accurately determine the cause of anaphylaxis and keep more detailed records of previous reactions. A limitation of this study is that all the data presented were collected retrospectively and thus prone to reporting bias.
The present study showed that anaphylaxis appeared to occur more often in adults than in infants and children in the Chinese population. The most common cause of anaphylaxis was food, particularly wheat. Anaphylaxis induced by fruits/vegetables was also important. Traditional Chinese medicine was the most common drug allergen. Epinephrine was administered in only a small number of cases. The current status of emergency treatment for anaphylaxis in China is not adequate.
Figures and Tables
Table 1
Triggers of 1,952 anaphylactic reactions

Table 2
Levels of specific IgE of 10 common food-triggers in food-induced anaphylactic reactions
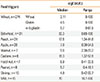
Table 3
Severity of anaphylaxis and potential risk factors
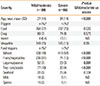
Table 4
Characteristics of patients with recurrent anaphylaxis (more than 10 episodes) before their visits

Table 5
Emergency treatment of 1,467 anaphylactic reactions

a, among 1,467 cases of total anaphylaxis; b, among 631 cases of mild/moderate anaphylaxis; c, among 836 cases of severe anaphylaxis; d, among 146 home-treated cases of anaphylaxis; e, among 107 home-treated cases of mild/moderate anaphylaxis; f, among 39 home-treated cases of severe anaphylaxis; g, among 640 ED-treated cases of anaphylaxis; h, among 227 ED-treated cases of mild/moderate anaphylaxis; i, among 413 ED-treated cases of severe anaphylaxis Bold denotes a significant P value.
n, number; ED, emergency department; ND, Not different.
ACKNOWLEDGMENTS
We thank Prof. Huang Xiaogu for revising the manuscript.
This study was supported by the Special Research Funds for Health Welfare Profession (No. 200802001) and the National Natural Science Foundation of China (No. 81273277).
References
1. Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010; 125:Suppl 2. S161–S181.
2. Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med. 2008; 101:139–143.
3. Huang F, Chawla K, Järvinen KM, Nowak-Węgrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012; 129:162–168.e1.
4. Ben-Shoshan M, La Vieille S, Eisman H, Alizadehfar R, Mill C, Perkins E, et al. Anaphylaxis treated in a Canadian pediatric hospital: Incidence, clinical characteristics, triggers, and management. J Allergy Clin Immunol. 2013; 132:739–741.e3.
5. Banerji A, Rudders SA, Corel B, Garth AM, Clark S, Camargo CA Jr. Repeat epinephrine treatments for food-related allergic reactions that present to the emergency department. Allergy Asthma Proc. 2010; 31:308–316.
6. Solé D, Ivancevich JC, Borges MS, Coelho MA, Rosário NA, Ardusso L, et al. Latin American Anaphylaxis Working Group. Anaphylaxis in Latin American children and adolescents: the Online Latin American Survey on Anaphylaxis (OLASA). Allergol Immunopathol (Madr). 2012; 40:331–335.
7. Shek LP, Lee BW. Food allergy in Asia. Curr Opin Allergy Clin Immunol. 2006; 6:197–201.
8. Yang MS, Lee SH, Kim TW, Kwon JW, Lee SM, Kim SH, et al. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008; 100:31–36.
9. Beyer K, Eckermann O, Hompes S, Grabenhenrich L, Worm M. Anaphylaxis in an emergency setting - elicitors, therapy and incidence of severe allergic reactions. Allergy. 2012; 67:1451–1456.
10. Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009; 123:434–442.
11. Smit DV, Cameron PA, Rainer TH. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005; 28:381–388.
12. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006; 47:373–380.
13. Adkinson NF, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF, et al. Middleton's allergy: principles and practice. eighth edition. Philadelphia: Elsevier;2014. p. 1119–1132.
14. Lauritano EC, Novi A, Santoro MC, Casagranda I. Incidence, clinical features and management of acute allergic reactions: the experience of a single, Italian Emergency Department. Eur Rev Med Pharmacol Sci. 2013; 17:Suppl 1. 39–44.
15. Hsin YC, Hsin YC, Huang JL, Yeh KW. Clinical features of adult and pediatric anaphylaxis in Taiwan. Asian Pac J Allergy Immunol. 2011; 29:307–312.
16. Lieberman P, Camargo CA Jr, Bohlke K, Jick H, Miller RL, Sheikh A, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006; 97:596–602.
17. Panesar SS, Javad S, de Silva D, Nwaru BI, Hickstein L, Muraro A, et al. The epidemiology of anaphylaxis in Europe: a systematic review. Allergy. 2013; 68:1353–1361.
18. Hompes S, Köhli A, Nemat K, Scherer K, Lange L, Rueff F, et al. Provoking allergens and treatment of anaphylaxis in children and adolescents--data from the anaphylaxis registry of German-speaking countries. Pediatr Allergy Immunol. 2011; 22:568–574.
19. Mehl A, Wahn U, Niggemann B. Anaphylactic reactions in children--a questionnaire-based survey in Germany. Allergy. 2005; 60:1440–1445.
20. Rolla G, Mietta S, Raie A, Bussolino C, Nebiolo F, Galimberti M, et al. Incidence of food anaphylaxis in Piemonte region (Italy): data from registry of Center for Severe Allergic Reactions. Intern Emerg Med. 2013; 8:615–620.
21. Worm M, Edenharter G, Ruëff F, Scherer K, Pföhler C, Mahler V, et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012; 67:691–698.
22. Cai PP, Yin J. Association between single nucleotide polymorphisms and wheat-dependent exercise-induced anaphylaxis in Chinese population. Chin Med J (Engl). 2013; 126:1159–1165.
23. Palosuo K, Varjonen E, Kekki OM, Klemola T, Kalkkinen N, Alenius H, et al. Wheat omega-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J Allergy Clin Immunol. 2001; 108:634–638.
24. Yin J, Wen LP. Wheat-dependent Exercise-induced Anaphylaxis Clinical and Laboratory Findings in 15 cases. Chin J Allergy Clin Immunol. 2010; 4:8.
25. Rudders SA, Banerji A, Vassallo MF, Clark S, Camargo CA Jr. Trends in pediatric emergency department visits for food-induced anaphylaxis. J Allergy Clin Immunol. 2010; 126:385–388.
26. Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in seoul. Allergy Asthma Immunol Res. 2014; 6:131–136.
27. Goh DL, Lau YN, Chew FT, Shek LP, Lee BW. Pattern of food-induced anaphylaxis in children of an Asian community. Allergy. 1999; 54:84–86.
28. Tai YS, JT Z, Jin GR. Investigation of aeroborne allergenic pollens in different regions of China. Beijing: Peking Publishing House;1991.
29. Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006; 61:461–476.
30. Ma S, Yin J, Jiang N. Component-resolved diagnosis of peach allergy and its relationship with prevalent allergenic pollens in China. J Allergy Clin Immunol. 2013; 132:764–767.
31. Cao HS, He PB, Yang LZ. Analysis of 288 anaphylaxis cases induced by herb injections. Chin J Pharmacoepidemio. 2006; 15:26–27.
32. Ye YM, Kim MK, Kang HR, Kim TB, Sohn SW, Koh YI, et al. Predictors of the severity and serious outcomes of anaphylaxis in Korean adults: a multicenter retrospective case study. Allergy Asthma Immunol Res. 2015; 7:22–29.
33. Mullins RJ. Anaphylaxis: risk factors for recurrence. Clin Exp Allergy. 2003; 33:1033–1040.
34. Morita E, Kunie K, Matsuo H. Food-dependent exercise-induced anaphylaxis. J Dermatol Sci. 2007; 47:109–117.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download