Abstract
Purpose
Nonallergenic irritants can aggravate the symptoms of rhinitis. We investigated the clinical responses of children with allergic rhinitis (AR) and nonallergic rhinitis (NAR) to nonallergenic irritants, and identified factors associated with these responses.
Methods
Children with chronic rhinitis (n=208) were classified as having AR or NAR based on the presence of aeroallergen-specific IgE. Healthy controls (n=24) were recruited for comparison. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines were used to classify patients, and their irritant score (0-21 points) and current symptom score (5-35 points) were measured. Subjects with irritant scores ≥3 and <3 were classified as having irritant and nonirritant rhinitis, respectively.
Results
The mean age of enrolled subjects was 6.8 years (range: 1.8-16.0 years). The AR and NAR groups had similar irritant scores (P=0.394) and proportions of subjects with irritant scores ≥3 (P=0.105). Irritant score correlated positively with symptom score (P=0.005), and the proportion of subjects with irritant scores ≥3 was greater in children with moderate-severe rhinitis than in those with mild rhinitis (P=0.046). Multiple logistic regression analysis indicated that the presence of atopic eczema increased the risk for sensitivity to a nonallergenic irritant (aOR=2.928, 95% CI 1.567-5.473, P=0.001).
Rhinitis is an inflammatory disease of the nose that is characterized by numerous nasal symptoms.1 Chronic or recurrent rhinitis, excluding cases caused by infection, is classified as allergic rhinitis (AR) or nonallergic rhinitis (NAR) depending on aeroallergen sensitization.2 Some studies have suggested that mixed rhinitis (MR), in which patients with AR experience nasal symptoms following nonallergenic irritants, is a distinct subtype of rhinitis.3456
Patients with AR and NAR may also show hyperreactivity to nonallergenic physical and chemical irritants, such as ammonia, cold air, cleaning products, newsprint, and chlorine.789 Such hyperreactivity is a clinical marker of increased nasal hyperresponsiveness.10111213 AR and NAR patients with more severe symptoms experience more severe responses to these nonallergenic irritants.12 However, information on patients with nasal hyperresponsiveness, in particular children, is limited. Given that the pediatric population has significant age-dependent variation in the prevalence of different types of rhinitis,14 the prevalence of MR in children of different ages requires investigation.2
The aims of the present study of children with AR and NAR were to: (1) investigate the responses to different nonallergenic irritants, (2) determine the effect of age on response to nonallergenic irritants in children with AR and NAR, and (3) identify clinical factors that influence rhinitis responses to nonallergenic irritants.
All patients with rhinitis who presented to the Allergy Section of the Pediatrics Department, CHA University Hospital (Seongnam, Korea) from November 2012 to January 2014 and met inclusion criteria were enrolled. These patients were children under 18 years of age and had chronic or recurrent rhinitis, defined as the presence of (1) episodes of rhinorrhea, nasal congestion, blockage, or attacks of sneezing, and nasal itching for at least 1 hour in the previous 12 months that were not attributable to cold or flu, and (2) symptoms that recurred during the past 2 years or throughout the past 3 months. Patients were excluded if they had conditions whose symptoms mimicked rhinitis15 or were suggestive of other immunologic diseases.16 Healthy subjects were recruited from the local community. Study subjects were stratified by age (0-4 years, 5-6 years, and 7-16 years) because the prevalence of allergic sensitization increases with age.
Skin prick tests were performed with the following allergens: Dermatophagoides D. pteronyssinus and D. farinae (house dust mites), tree pollen I and II, grass pollen, weed pollen, mold, and an animal mixture, all from Allergopharma (Allergopharma, Reinbeck, Germany). A child was deemed sensitized if at least 1 aeroallergen evoked a positive response (wheal diameter of at least 3 mm, and larger than the negative control at 15 minutes). AR was defined as the presence of a history of any rhinitis symptom and a positive test result for allergic sensitization.17 NAR was defined as the presence of any rhinitis symptom and a negative test result for allergic sensitization.2 We did not classify AR as seasonal or perennial because 90% of the Korean pediatric population with allergic sensitization is sensitized to house dust mites,18 and few are purely hay fever patients.
Rhinitis was classified as mild or moderate-severe and as intermittent or persistent according to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines.14
On the day of the hospital visit, the severity of rhinitis was measured by 5 symptoms—sneezing, runny nose (rhinorrhea), congestion (stuffiness), itchy nose, and postnasal drip during the previous 1 month—using an established 7-point visual analog scale for grading the severity of nasal symptoms of AR and NAR.15 For each symptom, a score of 1 means no symptoms or an occasional limited episode, and a score of 7 means unbearably severe symptoms that inhibit performance of tasks at all times. The total rhinitis symptom score was defined as the sum of these 5 symptom scores (total: 5-35 points).
We investigated situations that triggered allergic reactions, such as waking up in the morning, cleaning the house, visiting other people's homes, and physical contact with animals.19
All participants completed questionnaires that assessed their responses to nonallergenic irritants, which included the following 21 items: perfume, soap powders, hair spray, varnish, ammonia, mold odors, paints, saw dust, cigarette smoke, crude oil, solvents, bleach, pine tree odors, wood smoke, fresh newsprint, cosmetics, household cleaners, cooking odors, high air pollution, cold air, and weather change.320 Specifically, the parents of the children were asked 'Has your child shown any rhinitis symptoms (runny nose, nasal congestion, sneezing, or itching) when exposed to any of the 21 nonallergenic irritants?' They could answer "yes", "no", or "I do not know." Each positive answer was assigned 1 point; hence, the range was 0 to 21 points. Children with scores of 3 points or more were assigned to the irritant group and the others to the nonirritant group.
Differences between the AR and NAR groups and controls were assessed using Student's t test or the post hoc least significant difference (LSD) test for normally distributed data, and chi-square tests for categorical data. The distribution of irritant scores and symptom scores were examined for normality prior to applying statistical analysis using IBM SPSS Statistics for Windows, Ver. 24.0 (IBM Co., Armonk, NY, USA). A receiver operating characteristic (ROC) curve was then generated to find the optimal cutoff point for discrimination of rhinitis patients from controls. For analysis of factors that elicit responses, logistic regression analysis was performed with rhinitis as the dependent variable and age, sex, the presence of specific IgE, parental asthma, AR, atopic dermatitis and/or other diseases, pet ownership, housing type, exposure to second-hand smoke, and number of siblings as confounding variables. A P value of less than 0.05 was considered significant.
We enrolled 232 patients, 143 with AR (61.6%), 65 with NAR (28.0%), and 24 healthy controls (10.3%). Among the entire study population, 27.6% of subjects had asthma and 58.2% had atopic eczema. The overall incidence of parental asthma, AR, and atopic eczema was 6.0%, 59.9%, and 9.5%, respectively. The prevalence of AR increased with age (P=0.001), but the prevalence of irritant rhinitis did not (P=0.358) (Fig. 1). The irritant score and current symptom score were higher in the AR and NAR groups than in the control group (Table 1).
In the AR group, the number of patients with sensitization to house dust mites and poly-sensitization was 131 (91.6%) and 46 (32.2%), respectively, and these numbers were significantly greater than in the control group (both P<0.001). The most common nonallergenic irritants in the control group were cold air (n=16, 66.7%) and weather change (n=16, 66.7%). The most common nonallergenic irritants in the AR and NAR groups were cold air and weather change and their percentages were comparable between groups.
The AR and NAR groups differed significantly in the current symptom score (P=0.002), but not in the irritant score (P=0.394) (Fig. 2A and B). The mild rhinitis and moderate-severe rhinitis groups (classified by the ARIA guidelines) differed significantly in irritant score (P=0.046) and symptom score (P<0.001) (Fig. 2C and D). The intermittent rhinitis and persistent rhinitis groups did not differ significantly in irritant score (P=0.249), but did differ significantly in current symptom score (P=0.001) (Fig. 2E and F).
After adjusting for age and sex, multivariable regression analysis showed that "after waking up in the morning" (adjusted odds ratio [aOR]: 3.04, 95% confidence interval [CI]: 1.44-6.26, P=0.003), "cleaning the house" (aOR: 3.25, 95% CI: 1.14-9.30, P=0.028), "visiting other people's homes" (aOR: 2.61, 95% CI: 1.29-5.29, P=0.008), and "physical contact with animals" (aOR: 8.81, 95% CI: 1.80-43.14, P=0.007) were significantly associated with AR (Table 2).
ROC analysis indicated that the optimal cut-off point, i.e. the point that provided the best possible tradeoff between sensitivity and specificity (closest to the left-hand border and the top border of the ROC space), was an irritant score of 3 points (AUC=0.733) (Fig. 3).
The irritant response group (i.e. irritant score ≥3) constituted 74.1% (n=106) of the AR group and 63.1% (n=41) of the NAR group (P=0.105). The prevalence of sensitivity to different irritants was comparable in the AR and NAR groups (in decreasing order: weather change, cold air, high air pollution, cigarette smoke, and mold odors).
After adjusting for confounding factors that could influence response to nonallergenic irritants, the results indicated that atopic eczema (aOR: 3.05, 95% CI: 1.67-5.56, P<0.001) and parental atopic eczema (aOR: 3.44, 95% CI: 1.14-10.36, P=0.028) were significantly associated with increased risk for irritant rhinitis (Table 3).
The present findings indicate that children with AR and NAR had similar hyperreactivity to nonallergenic irritants. However, irritant score was significantly related to the severity of rhinitis in each group. Relative to NAR patients, AR patients had greater aggravation of rhinitis symptoms following allergenic triggers, such as waking up in the morning, cleaning the house, visiting other people's homes, and physical contact with animals, as expected. However, irritant rhinitis patients showed similar responses to these allergenic triggers as nonirritant rhinitis patients. The presence of atopic eczema in the patient or a family member played an important role in predicting irritant score. This suggests that individuals with atopic eczema are more likely to have severe reactions to nonallergenic irritants and allergenic triggers, presumably due to hyperreactivity of their nasal epithelia.
In our study, AR and NAR patients had similar hyperreactivity to nonallergenic irritants, suggesting that irritant score is not useful for differentiating AR from NAR. These findings agree with previous studies which demonstrated that adults with AR and NAR had no significant differences in nasal hyperreactivity to cold dry air,21 capsaicin challenge,22 and nonallergenic irritants.23 However, we found that irritant score correlated with current rhinitis symptom score in children with AR or NAR. This suggests that the score may be useful for classifing rhinitis patients according to disease severity. This correlation may be explained by the moderate correlation between nasal hyperreactivity and daily nasal symptoms in patients with perennial AR24 and an increase in nasal symptoms following exposure to irritants despite the lack of nasal priming to allergens.25 This may be because rhinitis often coexists with rhinosinusitis or adenoid hypertrophy. The sinus cavities are lined with a mucous membrane and directly connected to the nasal cavity and indirectly connected via systemic inflammation and the neurogenic reflex.1526 As nasal inflammation influences the sinus cavities,27 the existence of inflammation within the sinuses (likely from rhinitis or sinusitis) could prime circulating mediators to migrate to the nose or could trigger the nasal sensory afferent nerve. Although such responses may affect the response to irritants regardless of allergy,21 we did not collect data on comorbidities in the present study.
An irritant score of 3 or more was significantly correlated with more severe rhinitis. A response of 2 points or lower could be part of a normal functional response to cold air, mainly to preserve homeostasis and avoid mucosal dryness and damage.5 When we categorized children as having irritant rhinitis or non-irritant rhinitis, 74.1% of all children had MR. Some studies reported the percentages of MR among adults with AR were 42%12, 34%4, 52%3, and 40%.20 Thus, the prevalence of MR was higher in our study population. Other studies and ours found that AR and NAR patients have comparable responses to non-allergenic irritants in provocation tests.2122 Given that subjects with MR show symptoms that depend on their disease severity when exposed to irritant stimulants, it would be inaccurate to classify MR as a subgroup of AR. It seems more appropriate to consider patients traditionally defined as having MR as part of a larger group of patients who have sensitivities to nonallergenic irritants and may have either AR or NAR.5 The presence of intrinsic systemic inflammation must be verified for patients with irritant rhinitis to corroborate this classification. In our study, there was no significant effect of age on the irritant score or the prevalence of irritant rhinitis. Although a few studies suggested that nonspecific bronchial reactivity in the bronchial provocation test depends on age, the reason for this effect is unclear.28 Likewise, it is unclear whether nasal hyperreactivity depends on age in patients with rhinitis, a type of allergic airway disease.
The presence of atopic eczema in a patient and a family history of atopic eczema played an important role in predicting the irritant score. Such children were also more likely to have irritant rhinitis than nonirritant rhinitis. Individuals with atopic eczema may be more predisposed to more severe reactions to nonallergenic irritants and allergenic triggers due to hyperreactivity of their nasal epithelia.
We can suggest 2 possible explanations for the relationship between atopic eczema and irritant score. First, during development, the nose and skin both arise from ectodermal tissue, and tissues of common embryonic origin often have similar properties.29 Second, there may be heritable factors that predispose individuals to atopic eczema that is mediated by systemic inflammation cells, but not IgE, so that family members share a lower threshold for irritant responsiveness in the nose and other parts of the body.30 Patients with atopic eczema have a reduced threshold for pruritus,31 but atopic eczema is unrelated to the ability to produce IgE.32 Thus, it is possible that nasal hyperresponsiveness to nonallergenic irritants in patients with irritant rhinitis could be due to the presence of the atopic nose, an extension of the atopic skin.
The present study has some limitations. We did not classify NAR patients into subgroups.933 In contrast to adults, only a small proportion of children with NAR have eosinophilic rhinitis, localized AR, hormonal rhinitis, occupational rhinitis and drug rhinitis.34 Additional study is needed to identify clinical factors that coexist with rhinosinusitis or adenoid hypertrophy and that might influence the nasal responses to irritants in children with rhinitis. The current study population consisted of subjects who visited the pediatric outpatient allergy clinic at a tertiary referral hospital. Thus, these patients may not be representative of children with AR and NAR in the general population, possibly leading to an overestimation of the prevalence of AR. However, the ratio of AR to NAR in our study was 2.2:1, being consistent with those of previous epidemiologic studies of the general populations with rhinitis.4 Another limitation is that we utilized an irritant score questionnaire, which can be more subjective than nasal challenge testing in determining the severity of irritation.5 Also, use of a parent-based questionnaire may have led to some discrepancies, missing data, and recall bias.
In conclusion, we found that the irritant score was significantly associated with the severity of rhinitis in our population of children with AR and NAR, but that the score appears to be insufficient for differentiating AR from NAR. Unlike the pre-existing categorization, we found that individuals with AR and NAR both reacted to nonallergenic irritants. Such patients are more likely to have atopic eczema or a parent with atopic eczema. It is also possible that a patient with irritant rhinitis may have a unique underlying pathophysiology, in addition to or instead of the pathophysiology associated with AR and NAR, which could lead to an abnormally low threshold for reaction to irritants. This should be addressed in further research.
Figures and Tables
 | Fig. 1Age distribution of subjects with allergic rhinitis and nonallergic rhinitis (A) and irritant rhinitis and nonirritant rhinitis (B). The percentage of children with allergic rhinitis increased with age (P=0.001), but that of those with irritant rhinitis did not (P=0.358). |
 | Fig. 2(A-F) Total symptom scores (left column) and irritant scores (right column) in response to 21 different nonallergenic irritants in children with allergic rhinitis and nonallergic rhinitis (A and B), mild rhinitis and moderate-severe rhinitis (C and D), and intermittent rhinitis and persistent rhinitis (E and F). |
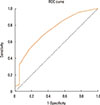 | Fig. 3Receiver operating characteristics (ROC) curve obtained from the sum of scores in response to 21 different nonallergenic irritants in the control and rhinitis groups. An irritant score between 2.5 and 3.5 provided the best compromise between sensitivity and (1-specificity) (sensitivity 68.8% and 51.2%, specificity 64.7% and 82.4%, respectively). Specificity was 94.1% at 4.5 points. |
Table 1
Demographic characteristics of allergic rhinitis and nonallergic rhinitis groups

Table 2
Responses to allergenic and nonallergenic irritant triggers in allergic rhinitis and nonallergic rhinitis patients

Table 3
Regression analysis of irritant score and other confounding factors

| OR (95% CI) | P value | |
|---|---|---|
| Parental atopic eczema | 3.44 (1.14-10.36) | 0.028 |
| Co-morbidity - atopic eczema | 3.05 (1.67-5.56) | <0.001 |
Odds ratio of irritant score being greater than or equal to 3 points; Irritant score as the dependent variable and age (months), gender, presence of sensitization (yes, no), parental asthma, AR or AD, co-morbidity with asthma or AD, pet-owning, housing type (apartment, villa, house), second-hand smoking (none, out of the house and in the house) and number of siblings (1 or 2 and more) as confounding factors.
References
1. Bousquet J, Fokkens W, Burney P, Durham SR, Bachert C, Akdis CA, et al. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy. 2008; 63:842–853.
2. Chiang WC, Chen YM, Tan HK, Balakrishnan A, Liew WK, Lim HH, et al. Allergic rhinitis and non-allergic rhinitis in children in the tropics: prevalence and risk associations. Pediatr Pulmonol. 2012; 47:1026–1033.
3. Bernstein JA, Levin LS, Al-Shuik E, Martin VT. Clinical characteristics of chronic rhinitis patients with high vs low irritant trigger burdens. Ann Allergy Asthma Immunol. 2012; 109:173–178.
4. Settipane RA. Demographics and epidemiology of allergic and nonallergic rhinitis. Allergy Asthma Proc. 2001; 22:185–189.
5. Baroody FM. How nasal function influences the eyes, ears, sinuses, and lungs. Proc Am Thorac Soc. 2011; 8:53–61.
6. Bernstein JA. Allergic and mixed rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2010; 31:365–369.
7. Dykewicz MS, Fineman S. Executive Summary of Joint Task Force Practice Parameters on Diagnosis and Management of Rhinitis. Ann Allergy Asthma Immunol. 1998; 81:463–468.
8. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.
9. Baraniuk JN. Pathogenic mechanisms of idiopathic nonallergic rhinitis. World Allergy Organ J. 2009; 2:106–114.
10. Hilberg O, Grymer LF, Pedersen OF. Nasal histamine challenge in nonallergic and allergic subjects evaluated by acoustic rhinometry. Allergy. 1995; 50:166–173.
11. Márquez F, Sastre J, Hernández G, Cenjor C, Sanchez-Hernandez JM, Sánchez J, et al. Nasal hyperreactivity to methacholine measured by acoustic rhinometry in asymptomatic allergic and perennial nonallergic rhinitis. Am J Rhinol. 2000; 14:251–256.
12. Shusterman D, Murphy MA. Nasal hyperreactivity in allergic and non-allergic rhinitis: a potential risk factor for non-specific building-related illness. Indoor Air. 2007; 17:328–333.
13. Shusterman DJ, Tilles SA. Nasal physiological reactivity of subjects with nonallergic rhinitis to cold air provocation: a pilot comparison of subgroups. Am J Rhinol Allergy. 2009; 23:475–479.
14. Bousquet J, Van Cauwenberge P, Khaltaev N. Aria Workshop Group. World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001; 108:S147–S334.
15. Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008; 122:S1–S84.
16. Subbarayan A, Colarusso G, Hughes SM, Gennery AR, Slatter M, Cant AJ, et al. Clinical features that identify children with primary immunodeficiency diseases. Pediatrics. 2011; 127:810–816.
17. Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy. 2011; 41:851–859.
18. Kim HY, Shin YH, Yum HY, Jee HM, Jang SJ, Yoon JW, et al. Patterns of sensitisation to common food and inhalant allergens and allergic symptoms in pre-school children. J Paediatr Child Health. 2013; 49:272–277.
19. Gendo K, Larson EB. Evidence-based diagnostic strategies for evaluating suspected allergic rhinitis. Ann Intern Med. 2004; 140:278–289.
20. Bernstein JA. Characteristics of nonallergic vasomotor rhinitis. World Allergy Organ J. 2009; 2:102–105.
21. Segboer CL, Holland CT, Reinartz SM, Terreehorst I, Gevorgyan A, Hellings PW, et al. Nasal hyper-reactivity is a common feature in both allergic and nonallergic rhinitis. Allergy. 2013; 68:1427–1434.
22. Sanico AM, Philip G, Proud D, Naclerio RM, Togias A. Comparison of nasal mucosal responsiveness to neuronal stimulation in non-allergic and allergic rhinitis: effects of capsaicin nasal challenge. Clin Exp Allergy. 1998; 28:92–100.
23. Bachert C. Persistent rhinitis - allergic or nonallergic? Allergy. 2004; 59:Suppl 76. 11–15.
24. de Graaf-in't Veld T, Koenders S, Garrelds IM, Gerth van Wijk R. The relationships between nasal hyperreactivity, quality of life, and nasal symptoms in patients with perennial allergic rhinitis. J Allergy Clin Immunol. 1996; 98:508–513.
25. Bacon JR, McLean JA, Mathews KP, Banas JM. Priming of the nasal mucosa by ragweed extract or by an irritant (ammonia). J Allergy Clin Immunol. 1981; 67:111–116.
26. Kim JH, Kim YS, Cho GS, Kim NH, Gong CH, Lee BJ, et al. Human rhinovirus-induced proinflammatory cytokine and interferon-β responses in nasal epithelial cells from chronic rhinosinusitis patients. Allergy Asthma Immunol Res. 2015; 7:489–496.
27. De Schryver E, Devuyst L, Derycke L, Dullaers M, Van Zele T, Bachert C, et al. Local immunoglobulin e in the nasal mucosa: clinical implications. Allergy Asthma Immunol Res. 2015; 7:321–331.
28. Mochizuki H, Shigeta M, Kato M, Maeda S, Shimizu T, Mirokawa A. Age-related changes in bronchial hyperreactivity to methacholine in asthmatic children. Am J Respir Crit Care Med. 1995; 152:906–910.
29. Bousquet J, Schunemann HJ, Fonseca J, Samolinski B, Bachert C, Canonica GW, et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy. 2015; 70:1372–1392.
30. Nassif A, Chan SC, Storrs FJ, Hanifin JM. Abnormal skin irritancy in atopic dermatitis and in atopy without dermatitis. Arch Dermatol. 1994; 130:1402–1407.
31. Leung DY. Pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1999; 104:S99–S108.
32. Camarda LE, Grayson MH. Can specific IgE discriminate between intrinsic and atopic asthma? Am J Respir Crit Care Med. 2011; 184:152–153.
33. Campo P, Rondon C, Gould HJ, Barrionuevo E, Gevaert P, Blanca M. Local IgE in non-allergic rhinitis. Clin Exp Allergy. 2015; 45:872–881.
34. Chawes BL, Kreiner-Møller E, Bisgaard H. Objective assessments of allergic and nonallergic rhinitis in young children. Allergy. 2009; 64:1547–1553.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download