Abstract
Purpose
Omalizumab is the preferred add-on therapy for patients with moderate-to-severe persistent allergic asthma and has demonstrated efficacy and safety in various ethnicities. This study evaluated the efficacy and safety of omalizumab in Chinese patients with moderate-to-severe allergic asthma.
Methods
This randomized, double-blind, parallel-group, placebo-controlled, phase III study assessed lung function, quality of life, asthma control, and safety of omalizumab after 24-week therapy in Chinese patients (18-75 years of age).
Results
A total of 616 patients were randomized (1:1) to omalizumab or placebo. The primary endpoint, least squares mean treatment difference (LSM-TD) in morning peak expiratory flow (PEF) (omalizumab vs placebo), at Weeks >20-24 was 8.85 L/min (Full analysis set; P=0.062). Per-protocol analysis set showed significant improvements with LSM-TD of 11.53 L/min in mean mPEF at Weeks >20-24 (P=0.022). The FEV1 % predicted was significantly improved with omalizumab vs placebo from 8 to 24 weeks (after 24-week treatment: LSM-TD=4.12%; P=0.001). At Week 24, a higher proportion of omalizumab-treated patients achieved clinically relevant improvements in standardized AQLQ (58.2% vs 39.3%; LSM=0.51 vs 0.10; P<0.001) and ACQ (49.5% vs 35.5%; LSM=-0.51 vs -0.34; P=0.002) scores vs placebo. Total and nighttime symptom scores reduced significantly with omalizumab vs placebo (LSM-TD=-0.21, P=0.048 and -0.12, P=0.011, respectively). Although the study was not powered to study differences in exacerbation rates (P=0.097), exacerbations in winter months were less frequent in the omalizumab vs placebo group (2 vs 21). Adverse event and severe adverse event rates were comparable between omalizumab and placebo.
Asthma is one of the most common chronic respiratory diseases that results in pulmonary inflammation and reversible lower airway obstruction in subjects of all age groups and is a major cause of socioeconomic implications in many countries.1234 Immunoglobulin E (IgE) plays a central role in inflammation by binding of its Fc region to FcεRI receptors on mast cells or FcεRII receptors on B lymphocytes and eosinophils to release inflammatory mediators.12
According to the Global Initiative for management of Asthma (GINA) burden report in 2004, the prevalence of asthma in China was 2.1%.5 The prevalence of asthma is increasing in China6 with a high case-fatality rate.7 The Chinese guidelines for asthma recommend oral corticosteroids (OCSs, lowest dose) and/or sustained-release theophylline as a step 5 therapy.8 Despite a high burden of pharmacotherapy, control of severe asthma is seldom achieved, and patients experience daily and nocturnal symptoms, frequent exacerbations, and poor quality of life, affecting daily activities.910
Omalizumab, a humanized monoclonal anti-IgE antibody, is recommended for the treatment of persistent moderate-to-severe allergic asthma.11 It binds to the Fc region of free-IgE, preventing its binding and allergen-induced cross-linking of high-affinity receptors on mast cells and basophils. With long-term therapy, high-affinity receptors on mast cells and basophils are down regulated, leading to a decrease in inflammatory mediators released.11213 Omalizumab has shown clinical benefits and favourable cost-effectiveness in patients with persistent severe allergic asthma and a high burden of disease.14 Considering evidence from randomized controlled clinical trials and real-world studies, the 2014 GINA asthma management guidelines suggested the use of add-on omalizumab as the preferred step 5 therapy for patients with uncontrolled allergic asthma.1516171819 However, there is no data on the management of Chinese patients with omalizumab. This study evaluates the efficacy, safety, and tolerability of omalizumab therapy, compared to placebo, in Chinese patients with moderate-to-severe persistent allergic asthma.
Patients were of either gender, aged 18-75 years, with confirmed diagnosis of moderate-to-severe persistent allergic asthma (inadequately controlled symptoms despite medium-to-high-dose ICS+LABA [GINA step 4] therapy) for ≥1-year duration at screening, weighing >20-≤150 kg, a serum total-IgE level of 30-700 IU/mL. Eligible patients had a documented positive reaction to at least 1 perennial aeroallergen and reported ≥2 or ≥3 exacerbation events in previous 12 or 24 months, respectively. Other inclusion criteria were forced expiratory volume in 1 second (FEV1) of 40%-80% of predicted normal with post-bronchodilator reversibility of ≥12% within 30 minutes and compliance with completion of peak expiratory flow (PEF)/electronic diary (eDiary) during the run-in period.
Patients were excluded if they had a history of malignancy, hypersensitivity, or severe food- or drug-related anaphylaxis, active lung disease other than allergic asthma, clinically significant ECG or chest X-ray abnormality, elevated total serum IgE level without increase in specific IgE, or use of other investigational drugs within at least 30 days/5 half-lives of enrolment.
This is a randomized, double-blind, placebo-controlled, and parallel-group study (Fig. 1A). Patient's eligibility criteria were assessed in a pre-treatment screening consisting of a 2-week optimization period and a 4-week run-in period (baseline) to determine patient's degree of asthma control. At the baseline visit, eligible patients were randomized (1:1) to receive either add-on omalizumab or add-on placebo by subcutaneous injections for 24 weeks. The dose and dosing frequency were determined by the omalizumab dosing table on the basis of patient's serum total-IgE level and body weight (≥0.016 mg/kg/IgE-IU/mL every 4 weeks). Patients were advised to use rescue medication (inhaled salbutamol) as needed and record it in the eDiary. The study was conducted in accordance with the current Good Clinical Practice, and the protocol was approved by Independent Ethics Committee or Institutional Review Board for each center. Written informed consent was obtained from each enrolled patient. The study was registered at http://clinicaltrials.gov (NCT01202903).
The efficacy and safety of add-on omalizumab treatment was assessed at 4-week (±3 days) intervals during the 24-week treatment period.
The primary efficacy objective was to assess the mean change from baseline in morning PEF (mPEF, L/min) measured using a PEF meter after 24 weeks of treatment. Patients were instructed to record their mPEF within 15 minutes of waking, before using any medication. The secondary endpoints included FEV1 % predicted, Asthma Control Questionnaire (ACQ) scores, asthma symptom scores and rescue medication use, standardized Asthma Quality of Life Questionnaire (AQLQ[S]) scores, Global Evaluation of Treatment Effectiveness (GETE) responder analysis, and safety. FEV1 % predicted at defined time-points and ACQ scores at Weeks 16 and 24 (Supplementary section S4) were assessed. Patients recorded the daily symptom scores (morning, daytime, and nocturnal symptoms) and rescue medication use twice daily (puffs taken in the last 12 hours; Supplementary section S6) using an eDiary. Investigators and patients assessed the GETE on a 5-point scale (excellent, good, moderate, poor, or worsening) at Weeks 16 and 24. AQLQ(S) at the beginning and after 24-week treatment period was used to assess the change in quality of life of patients (Supplementary section S5). The rate and seasonal effect of protocol-defined asthma exacerbations (clinically significant worsening of asthma requiring addition or increase in dose/dosing-frequency of systemic corticosteroids or intravenous theophylline) were assessed as exploratory outcomes.
All efficacy variables were analyzed, unless specified, using the full analysis set (FAS). FAS consisted of all patients who received ≥1 dose of the study drug. The per-protocol set (PPS), a subset of the FAS without major protocol deviations (Supplementary section S2), was used for supportive analysis to assess robustness of the primary analysis. The primary outcome, change from baseline to Week 24 in mPEF, was assessed using an analysis of covariance (ANCOVA) model with the terms (treatment arm, dosing schedule [every 2 and/or 4 weeks], gender, center grouping, smoking status), and baseline mPEF as the covariates. The changes from baseline of FEV1 % predicted, AQLQ(S) and ACQ scores at the end of the 24-week treatment period were analyzed using ANCOVA, with baseline values as covariates. The investigator's and patient's GETE were analyzed using the Cochran-Mantel-Haenszel test for treatment comparison with respect to responder rate (proportion of patients achieving excellent/good response). The change from baseline of total asthma symptom score was calculated as the total of each day's morning, daytime, and nocturnal scores, and treatment comparisons were evaluated using ANCOVA and the Van-Elteren test. Two-sided hypothesis tests were conducted at an alpha level of 0.05 for all endpoints. The analyses of changes from baseline in mean mPEF and FEV1 % predicted were based on the observed data. For mPEF if fewer than 7 daily readings during a 28-day period were available, the mean mPEF for that period were set to missing. Missing data was handled similarly for asthma symptom score. For ACQ and AQLQ(S), missing items were imputed by interpolation and for scoring ≤1 missing item and ≤10% of data was allowed, respectively.
All safety variables were analyzed using safety set unless specified. The safety set included all patients who received at least 1 dose of the study drug.
Among 1,480 patients screened, 616 eligible across 42 study centers in China were randomized (1:1) to either add-on omalizumab or placebo. Of these, 7 were screen failures, but randomized in the IRT system according to the investigative site, and excluded from the FAS and the safety set. One patient did not receive study medication for >60 days and was also removed from the FAS. In effect, 608 and 609 patients were included in the FAS and the safety set. PPS analysis included 536 patients, and details of protocol deviations leading to exclusion from the PPS dataset is provided in Supplementary section S2. Patient disposition is discussed in Fig. 1B. Both treatment arms were well-balanced in demographic and baseline characteristics (Table 1).
Omalizumab showed numerically greater improvement change from baseline in the mean mPEF after 24-week treatment when compared to placebo. However, the treatment difference vs the placebo group at Week 24 was not statistically significant (LSM treatment difference [LSM-TD]=8.85 L/min; P=0.062; Fig. 2A). At Weeks >4-8 through >16-20, omalizumab significantly improved mean mPEF from baseline vs placebo. PPS analysis showed significant improvements in mean mPEF with omalizumab vs placebo from Weeks >4-8, which persisted throughout the remainder of study duration (Week 24 LSM-TD=11.53 L/min; P=0.022; Fig. 2A).
The FEV1 % predicted at all time-points, from 8-24 weeks of treatment, was significantly improved with omalizumab vs placebo (after 24 weeks: LSM-TD=4.12%; P=0.001; Fig. 2B).
LSM changes from baseline of ACQ scores were clinically meaningful (reduction of ≥0.5 unit) in the omalizumab group (-0.51) at Week 24. Compared to placebo omalizumab showed a significant reduction in ACQ scores at Weeks 16 (LSM-TD=-0.20, P<0.001) and Week 24 (LSM-TD=-0.17, P=0.002; Fig. 3). The proportion of patients achieving clinically meaningful improvements in ACQ scores was 49.5% (104/210) for the omalizumab group vs 35.5% (75/211) for the placebo group when considering the response rate based on number of patients with available ACQ data. Approximately 30% of patients had missing ACQ data.
Statistically significant reductions were observed for total and night-time symptom scores with omalizumab vs placebo at Week 24 (LSM-TD=-0.21; P=0.048, -0.12, and P=0.011, respectively) (Fig. 4A). Use of rescue medication during day/night decreased (LSM-TD daytime/nighttime=-0.16/-0.14 puffs/day; Fig. 4B), and the percentage of days with no rescue medication increased significantly with omalizumab vs placebo (LSM±SEM=28.5±11.33 vs 23.3±11.44; LSM-TD=5.16 [95% CI: 0.14, 10.18]; all P<0.05).
After 16-week treatment, the proportion of patients who responded to treatment was significantly higher in the omalizumab group than in the placebo group (investigator's and patient's GETE being 71.9% vs 52.3%; P<0.001 and 70.6% vs 59.6%; P=0.006, respectively). Similarly at Week 24, investigator's and patient's GETE for omlaizumab vs placebo were 70.3% vs 50.7% (P<0.001) and 71.9% vs 61.6 (P=0.006), respectively (Supplementary Figure).
LSM changes from baseline for the overall (0.51), emotions (0.64), and environmental scores (0.54) were clinically meaningful (≥0.5-point change from baseline) in the omalizumab group. After 24-weeks of treatment, omalizumab significantly improved overall AQLQ(S) and all individual domain scores (Fig. 5) vs placebo. The proportion of patients achieving a clinically meaningful AQLQ(S) improvement was significantly higher (P<0.001) with omalizumab (58.2%, 106/182) vs placebo (39.3%, 2/178) for all evaluable patients. Of note, approximately 40% of patients had missing AQLQ(S) data.
The proportion of patients reporting asthma exacerbations was lower in the omalizumab vs placebo group (7.2% vs 10.9%, rate ratio=0.61; P=0.097). Protocol-defined exacerbations were less frequently reported in the omalizumab vs placebo groups in winter (2 vs 21), whereas for other seasons the frequency was comparable (for spring [8 vs 8], summer [8 vs 10], and autumn [9 vs 12]). Exacerbation rates per year were 0.06 vs 0.06 during spring, 0.06 vs 0.07 during summer, 0.06 vs 0.09 during autumn, and 0.01 vs 0.15 during winter, respectively, for omalizumab vs placebo.
Patients were exposed to a median duration of 169 days in both the study arms over 24 weeks. Patients received omalizumab or placebo every 2 or 4 weeks to reach a total of 6 or 12 doses, respectively. The overall incidence of AEs during the study period was similar in the omalizumab (39%) and placebo (39.5%) groups (Table 2). The majority of AEs in both groups were mild or moderate in severity. The incidence of severe AEs was lower in the omalizumab group (1.9%) vs the placebo group (3.0%) and similar for mild (omalizumab 29.0% vs placebo 27.4%) and moderate (15.8% vs 15.4%) AEs. Only 1 death was reported in the omalizumab group. The patient had 2 episodes of asthma exacerbation in 4 months before randomization and a declining PEF during the run-in period. No rescue medication use was reported during the 12 days before the exacerbation event. The cause of death was recorded as severe asthma exacerbation.
Omalizumab is the only humanized anti-IgE monoclonal antibody approved as an add-on therapy for patients with allergic asthma inadequately controlled with high-dose ICSs with or without other controller medication.20 Omalizumab has demonstrated efficacy and safety in numerous controlled trials and real-world studies, mostly in predominantly Caucasian populations.11212223242526272829
This is the first study investigating the efficacy and safety of omalizumab specifically in Chinese patients with moderate-to-severe persistent allergic asthma. Though the primary endpoint of demonstrating the effect of add-on omalizumab treatment on change from baseline in mean mPEF after 24 weeks of treatment vs placebo was not met in this study, statistically significant improvements in mPEF were observed with omalizumab vs placebo from Week 4-20. The statistically insignificant primary endpoint at Week 24 may have been affected by the variability observed in mPEF data, which increased over time in both treatment groups and peaked at Week 24. This variability may reflect the imprecision of a self-administered and effort-dependent test. It is worthwhile to note that the supportive analysis using the PPS, which excluded patients with major protocol deviations, showed that omalizumab was superior to placebo in improving the mean mPEF, starting at >4-8 weeks and persisting throughout the remainder of the 24-week treatment period (LSM-TD=11.53 L/min; P=0.022). Previously reported clinical studies in patients with allergic asthma have also shown improvements of 10-30 L/min in mPEF from their baseline values after 16-week treatment with omalizumab.262730 In the present study, evident from FEV1 % predicted values from Week 8-24, improvements in lung function observed with omalizumab is consistent with recent published results.1119212327
In addition to lung function, omalizumab has previously shown clinically relevant and significant improvements in the ACQ scores in patients with asthma vs placebo or optimized asthma therapy.1819283031 Improvements in ACQ scores (clinically relevant change from baseline and significant vs placebo), overall symptoms scores and decreased rescue medication use were observed with omalizumab in Chinese patients. Similar to results published in a previous study,18 investigator's and patient's GETE responder analysis (at Week 16 assessment) also showed a greater number of responders in the omalizumab vs placebo groups.
Omalizumab also significantly improved quality of life vs placebo, with respect to the overall AQLQ(S) scores as well as its individual domains. Recent real-life evidence in other populations also suggests an improvement in the quality of life of patients treated with omalizumab.192132
Previous studies have reported a higher mortality due to asthma in the winter season because of an increased incidence of seasonal influenza infection accompanied by severe asthma exacerbations.3334 Although the present study of Chinese patients was not powered for exacerbations, omalizumab reduced the exacerbation rate per year and the number of exacerbations in winter compared to placebo (0.01 vs 0.15 and 2 vs 21, respectively).
The majority of adverse events were mild or moderate in severity. One death in the omalizumab group occurred on the day after the first and only dose from severe asthma exacerbation. Though the investigator suspected the death to correlate with treatment, anaphylaxis was not reported to be the reason. No deaths were reported in the omalizumab groups in global pivotal studies.15163536
The international asthma management guidelines1120 (GINA and NHLBI) recommend omalizumab as a step 5 asthma therapy for moderate or severe persistent allergic asthmatic patients. Various studies have shown omalizumab to be an effective and safe therapy for moderate-to-severe allergic asthma in patients of Asian ethnicity outside China.5192627 This study demonstrated an improvement in lung function and symptoms related to asthma as well as decline in the frequency of daytime and nighttime symptoms, rescue medications use, or asthma exacerbations in Chinese patients who were treated with omalizumab. This resulted in enhanced quality of life in patients with moderate-to-severe persistent allergic asthma in this study. Additionally, despite one death, omalizumab presented a safety profile that was comparable with that of placebo.
Although no formal hypothesis testing has been performed, by informal comparison with various other studies including non-Chinese ethnicities, the results from this study in Chinese patients suggests no clinically relevant ethnic differences in response to omalizumab therapy.162627 Our results suggest that omalizumab may show similar tolerability and efficacy in Chinese patients compared to non-Chinese or Caucasian patients.
There are several limitations to this study. First, there is missing data for some variables (e.g., ACQ and AQLQ[S]) with interpolation performed. While expected to decrease precision around the point estimates, the fact that statistically significant differences between treatment groups were observed argues against these missing data as being a critical limitation. Secondly, this study is not powered for studying effects of omalizumab on asthma exacerbations. Thirdly, there is a lack of adjustment for multiple comparisons between secondary endpoints in this study. The fact that the benefits that were observed in these secondary end-points are consistent with those of previous trials1516171819 militates against concerns around lack of adjustment for multiple comparisons.
In conclusion, add-on treatment with omalizumab may prove to be an effective and safe therapy option for Chinese patients with moderate-to-severe persistent allergic asthma.
Figures and Tables
Fig. 1
(A) Study design and (B) patient disposition. One administrative problem in the omalizumab group was due to drug administration being performed incorrectly. For the placebo group, 4 administrative problems were due to incorrect administration of omalizumab instead of placebo. The above cases were considered as protocol deviation.
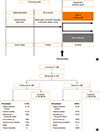
Fig. 2
Analysis of lung function following 24 weeks of treatment. (A) mean mPEF (L/min), (B) FEV1 % predicted. Data is presented as LSM-TD of omalizumab vs placebo for mPEF and LSM treatment values±SEM for FEV1 % predicted. FAS, full analysis set; FEV1, forced expiratory volume in 1 second; LSM-TD, least square mean-treatment difference; PP, per-protocol set; PEF, peak expiratory flow; SEM, standard error of mean.

Fig. 3
Analysis of changes from baseline in ACQ scores (full analysis set). Data is presented as LSM values±SEM from full analysis set. Patients with not more than 1 item missing are included with the missing item being imputed by interpolation (approximately 30% of patients in both treatment groups had missing ACQ data). ACQ, asthma control questionnaire; LSM, least squares mean; SEM, standard error of mean.

Fig. 4
Analysis of changes from baseline following 24 weeks of treatment in (A) asthma symptom scores and (B) asthma rescue medication. Data is presented as LSM treatment values±SEM from the full analysis set. LSM, least squares mean; SEM, standard error of mean.

Fig. 5
Analysis of change from baseline in the AQLQ(S) scores (full analysis set). Data is presented as LSM treatment values±SEM from the full analysis set. Patients with not more than 1 item missing are included with the missing item being imputed by interpolation (approximately 40% of patients in both the treatment groups had missing AQLQ(S) data). AQLQ(S), asthma quality of life questionnaire; LSM, least squares mean; SEM, standard error of mean.

Table 1
Demographic and baseline patient characteristics (safety set)
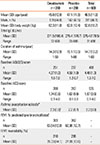
*Asthma exacerbation history of at least prior 12 months was collected during the screening period; †n=309; ‡data from the full analysis set.
ACQ, asthma control questionnaire; AQLQ(S), standardized asthma related quality of life questionnaire; FEV1, forced expiratory volume in 1 second; SD, standard deviation.
Table 2
Number of patients with AEs by preferred term (safety set)

ACKNOWLEDGMENTS
This study was supported by Novartis Pharma AG, Basel, Switzerland.
The authors acknowledge all investigators and patients who contributed to the study.
The authors would also like to thank Vasavi Yada and Jenni Gray (Novartis) for medical writing assistance.
Notes
References
1. Holgate ST, Djukanović R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005; 35:408–416.
2. Platts-Mills TA. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001; 164:S1–S5.
3. Ye YM, Kim SH, Hur GY, Kim JH, Park JW, Shim JJ, et al. Addition of montelukast to low-dose inhaled corticosteroid leads to fewer exacerbations in older patients than medium-dose inhaled corticosteroid monotherapy. Allergy Asthma Immunol Res. 2015; 7:440–448.
4. Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014; 6:376–388.
5. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.
6. Li J, Wang H, Chen Y, Zheng J, Wong GW, Zhong N. House dust mite sensitization is the main risk factor for the increase in prevalence of wheeze in 13- to 14-year-old schoolchildren in Guangzhou city, China. Clin Exp Allergy. 2013; 43:1171–1179.
7. Li XY, Hu N, Huang ZJ, Jiang Y, Wu F. Mortality and death cause proportion of respiratory disease in China, 2004-2005. Zhonghua Yu Fang Yi Xue Za Zhi. 2010; 44:298–302.
8. Asthma Workgroup. Chinese Thoracic Society. Chinese Societ of General Practitioners. Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version). J Thorac Dis. 2013; 5:667–677.
9. Chipps BE, Zeiger RS, Borish L, Wenzel SE, Yegin A, Hayden ML, et al. Key findings and clinical implications from the Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2012; 130:332–342.e10.
10. López Tiro JJ, Contreras EA, del Pozo ME, Gómez Vera J, Larenas Linnemann D. Real life study of three years omalizumab in patients with difficult-to-control asthma. Allergol Immunopathol (Madr). 2015; 43:120–126.
11. Song WJ, Cho SH. Challenges in the management of asthma in the elderly. Allergy Asthma Immunol Res. 2015; 7:431–439.
12. Inführ D, Crameri R, Lamers R, Achatz G. Molecular and cellular targets of anti-IgE antibodies. Allergy. 2005; 60:977–985.
13. Nam YH, Kim JH, Jin HJ, Hwang EK, Shin YS, Ye YM, et al. Effects of omalizumab treatment in patients with refractory chronic urticaria. Allergy Asthma Immunol Res. 2012; 4:357–361.
14. Sullivan SD, Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008; 63:670–684.
15. Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011; 154:573–582.
16. Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005; 60:309–316.
17. Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med. 2008; 102:1371–1378.
18. Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011; 66:671–678.
19. Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the 'real-world' effectiveness of omalizumab in allergic asthma. Respir Med. 2013; 107:1141–1151.
20. National Heart, Lung, and Blood Institute (US). Guidelines for the diagnosis and management of asthma (EPR-3) [Internet]. Bethesda (MD): National Heart, Lung, and Blood Institute;2007. 08. cited 2015 Jan 20. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines.
21. Barnes N, Menzies-Gow A, Mansur AH, Spencer D, Percival F, Radwan A, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013; 50:529–536.
22. Cazzola M, Camiciottoli G, Bonavia M, Gulotta C, Ravazzi A, Alessandrini A, et al. Italian real-life experience of omalizumab. Respir Med. 2010; 104:1410–1416.
23. Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013; 42:1224–1233.
24. D'Urzo AD, Wong J. Effectiveness of omalizumab in severe persistent asthma under real-life conditions. Can Fam Physician. 2014; 60:643–645.
25. Novelli F, Latorre M, Vergura L, Caiaffa MF, Camiciottoli G, Guarnieri G, et al. Asthma control in severe asthmatics under treatment with omalizumab: a cross-sectional observational study in Italy. Pulm Pharmacol Ther. 2015; 31:123–129.
26. Ohta K, Miyamoto T, Amagasaki T, Yamamoto M, Study G. 1304 Study Group. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology. 2009; 14:1156–1165.
27. Ohta K, Yamamoto M, Sato N, Ikeda K, Miyamoto T. One year treatment with omalizumab is effective and well tolerated in Japanese Patients with moderate-to-severe persistent asthma. Allergol Int. 2010; 59:167–174.
28. Schumann C, Kropf C, Wibmer T, Rüdiger S, Stoiber KM, Thielen A, et al. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J. 2012; 6:215–227.
29. Subramaniam A, Al-Alawi M, Hamad S, O'Callaghan J, Lane SJ. A study into efficacy of omalizumab therapy in patients with severe persistent allergic asthma at a tertiary referral centre for asthma in Ireland. QJM. 2013; 106:631–634.
30. Tajiri T, Niimi A, Matsumoto H, Ito I, Oguma T, Otsuka K, et al. Comprehensive efficacy of omalizumab for severe refractory asthma: a time-series observational study. Ann Allergy Asthma Immunol. 2014; 113:470–475.e2.
31. Braunstahl GJ, Chlumský J, Peachey G, Chen CW. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real-world setting. Allergy Asthma Clin Immunol. 2013; 9:47.
32. Brusselle G, Michils A, Louis R, Dupont L, Van de Maele B, Delobbe A, et al. "Real-life" effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009; 103:1633–1642.
33. Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006; 61:722–728.
34. Wark PA, Gibson PG. Asthma exacerbations. 3: pathogenesis. Thorax. 2006; 61:909–915.
35. Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001; 18:254–261.
36. Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001; 108:184–190.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download