Abstract
Purpose
Several studies over the past 4 decades have indicated a significant reduction in house dust mite (HDM) and HDM allergen concentration in areas higher than 1,500 m above sea level. These have served as basis of allergen avoidance therapies for HDM allergy and asthma. However, modern construction techniques used in the insulation, heating, and glazing of buildings as well as global warming have changed the environmental parameters for HDM living conditions. The present study revisits the paradigm of decreasing HDM allergen concentrations with increasing altitude in the alpine region of Germany and Austria.
Methods
A total of 122 dust samples from different abodes (hotels, privates and mountain huts) at different altitudes (400-2,600 m) were taken, and concentrations of HDM allergens were analyzed. Humidity and temperature conditions, and numerous indoor environmental parameters such as fine dust, type of flooring, age of building, and frequency of cleaning were determined.
Results
HDM allergen concentrations did not significantly change with increasing altitude or relative humidity. At the level of indoor parameters, correlations could be found for different flooring types and the concentration of HDM allergens.
Conclusions
In contrast to the widespread view of the relationship between altitude and HDM allergen concentrations, clinically relevant concentrations of HDM allergens could be detected in high-lying alpine regions in Austria and Germany. These results indicate that improvement in conditions of asthmatic patients sensitized against HDMs during a stay at high altitude can no longer be ascribed to decreased levels of HDM allergens, instead, other mechanisms may trigger the beneficial effect.
Asthma and allergic rhinitis are chronic diseases with a steadily increasing prevalence. Worldwide, 300 million people suffer from asthma. In Germany alone, 7,000 people die because of asthma each year. The mortality rate is rising in most countries, and allergy and asthma have become public health problems throughout the world.1
In principle, the allergic immune response results from a misguided immune polarization against proteins and other harmless biomolecules. Allergic sensitization is complex and multifactorial, including a genetic predisposition to mount IgE responses (atopy), exposure to microbial diversity, allergens and environmental pollutants, and even use of antibiotics in infancy.23 Moreover, protein-inherent features, such as protease activity (Der p 1) or MD2 mimicry (Der p 2), contribute to the "allergenicity" of certain molecules.456
HDM allergens are one of the main causes of allergy and asthma, and increased exposure to indoor allergens has been suggested as a possible reason for the general rise in allergic diseases.78910 The most frequent species are Dermatophagoides pteronyssinus (with the major allergens Der p 1 and Der p 2) and Dermatophagoides farinae (with the major allergens Der f 1 and Der f 2). Both comprise mite "group 1" and "group 2" allergens,11 and are common in European and American homes. Usually, HDMs colonize every part of a house, and HDM allergens are chemically stable for many years.1213 The structure and sequence of Der p 1 and Der f 1 are almost identical, thus resulting in a high cross-reactivity and similar effects in patients.14
The concentration of HDM allergens correlates with clinical markers for asthma severity, e.g. peak expiratory flow (PEF) variability, forced expiratory volume in 1 second (FEV1), or bronchial hyperresponsiveness (BHR) in asthmatic patients sensitized to HDMs.15 In general, the higher the allergen exposure, the more patients have positive skin prick tests, but even exposure to lower levels of HDM allergens (20 ng/g) was found to be a significant risk factor for sensitization.16 Since early publications in the 1970s, allergologists have postulated decreased HDM load and HDM allergen concentration with increasing altitude and decreasing humidity,17181920212223 while some publications contradict this interpretation.24252627 Based on the assumption of a negative correlation of altitude and HDM concentration, allergen avoidance therapies for HDM allergy and asthma are offered in alpine regions. High-altitude climate therapy has been shown to positively affect medical parameters and symptoms of patients suffering from allergic airway diseases. Clinical studies verified that a stay at high altitude improves relevant surrogate parameters for allergy and asthma, such as the reduction in specific IgE levels, improved clinical asthma markers,28 or mitigate nonspecific BHR and epithelial shedding.152930313233
As a second major parameter, relative air humidity (RH, %) was postulated to significantly influence HDM growth, and low indoor RH was recommended to reduce HDM.22333435 However, in recent studies no association between HDM infestation and RH could be detected.363738
Indoor allergens are usually attached to the surface of indoor particulate matter (PM), e.g. fine dust, which supports their transport to the respiratory tract and also acts as a bronchial irritant,39 which may also aggravate the allergenicity of indoor allergens and was therefore measured in all buildings included in this study.4041
The aim of the present study was to revisit the current hypothesis that altitude inversely correlates with the HDM allergen concentration. For that purpose, HDM allergen concentrations were measured at different heights in alpine regions of Austria and Germany, and these data were analyzed in terms of fine dust pollution, temperature, humidity, and parameters of building characteristics and indoor environment.
A total of 122 dust samples (65 samples below and 57 samples above 1,500 m a.s.l.) were collected from different types of buildings, such as private residences, taverns, and mountain huts, located at altitudes between 400 m and 2,600 m a.s.l in alpine regions of Germany and Austria. All samples were taken during September in 2012 and 2014, which has been reported as the month with the highest concentrations of mites measured in house dust,1942 using a DUSTREAM™ Collector with inserted nylon filter (INDOOR biotechnologiesINC, Charlottesville, VA, USA) attached to a vacuum cleaner. According to the INDOOR biotechnologiesINC protocol, in all 4 corners of a room, as well as a mattress, an area of 21×30 cm was vacuumed for 30 seconds. The samples collected were stored at -20℃ in dust-proof Ziploc bags (Toppits® Ziploc® Vaughan, ON, Canada).
Allergens were extracted by adding 2 mL of phosphate buffered saline (PBS) to the dust samples, shaking (at RT/30 min), spinning (3 min/4,000 rpm) and using 50 µL of the supernatant for further measurement. Allergen concentrations were measured using a MARIA 5-plex Multiplex Array for Indoor Allergens (MRA-C5) kit (INDOOR biotechnologiesINC) on a Luminex S-100 (Luminex, Austin, TX, USA) according to the manufacturers' instructions. Concentrations of Der p 1, Der f 1, and allergens of mite group 2 (both Der p 2 and Der f 2) were calculated via polynomial fit of raw fluorescent units into a dilution curve of allergen standards using xPONENT® software and expressed in ng/µL. The limit of detection was 0.06 ng/mL each for Der p 1 and Der f 1, and mite group 2 allergens are detectable up to 0.02 ng/mL.43 We investigated the weight (g) of collected dust of each sample and standardized the concentrations to nanogram allergen per gram of dust (ng/g). Spearman's rho correlation of allergen concentration with altitude, temperature, fine dust, and humidity was calculated using SPSS software (SPSS Inc., Chicago, IL, USA, version 22.0). Comparing of means was done with the Mann-Whitney U Test for independent samples. The relationship of indoor environmental characteristics with the concentration of allergens was calculated using univariate ANOVA and the post hoc Bonferroni test. The limit of significance was set to 0.05.
Particulate matter (PM-1, PM-2.5 and PM-10 in µg/m3), temperature, and RH% were measured with a Grimm Model 1.108 Portable Aerosol Spectrometer (Grimm, Aerosol Technik GmbH Co, Ainring, Germany). The spectrometer was positioned at a height of 50 cm in the middle of each room, and a ventilator was arranged for air circulation. The period of measurement covered about 5 minutes per room. The individual values were averaged arithmetically.
In addition, the position of each building was determined via GPS, and data concerning indoor environmental parameters (number of windows, type of glazing, type of flooring, age of building, quantity of linens, type of heating, people per day/night, floor number, number of beds, type and intensity of usage, cleaning method, and frequency) were collected and stored in an iPad® database system (Tap Zapp Software Inc., Calgary, Canada).
All allergens (Der p 1, Der f 1, and mite group 2) were detectable in 100% of the 122 dust samples collected. The concentration of mite allergens showed high variations. Mite group 2 ranged from 0.01 to 229.12 ng/g (arithmetic mean: 24.49±36.49 ng/g). Der p 1 levels varied from 0.03 to 426.90 ng/g (arithmetic mean: 32.59±63.16 ng/g), and Der f 1 levels ranged from 0.08 to 1,703.69 ng/g (arithmetic mean: 95.04±226.42 ng/g) (Fig. 1). Normal distribution tests failed.
No correlation could be measured between concentrations of the allergens Der p 1 and mite group 2 with the altitude (Der p 1: r=0.088, P=0.334; mite group 2: r=-0.098, P=0.285). Der f 1 showed a weak negative correlation with the altitude (Der f 1: r=-0.319/P<0.001), which may have been due to a markedly higher concentration of Der f 1 in the altitude range below 1,500 m compared to Der p 1 allergen levels (P=0.001). There was a huge percentage disparity of Der p 1 and Der f 1 allergens below 1,500 m altitude (Fig. 2). Concentrations of Der p 1 and Der f 1 allergens did not differ in samples taken above 1,500 m a.s.l. (P=0.689).
No statistically significant difference could be found between allergen concentrations of samples taken above and below 1,500 m a.s.l. of both Der p 1 (P=0.928) and mite group 2 allergens (P=0.127) (Fig. 3). Only Der f 1 was reduced in higher altitude (P<0.001).
In general, mattresses represent a superior biotope for HDM compared to floors. Consistently, allergen concentrations of samples collected from mattresses were significantly higher than those taken from the floors (Der p 1: P=0.001; Der f 1: P=0.005; mite group 2: P=0.017) reflecting a stoichiometric relationship of ~ 2:1 (Fig. 4).
One part of the samples (n=37) was taken from buildings that were only used 6 months a year, at an altitude of 1,750-2,600 m and fully frozen during winter. The other part (n=85) originated from buildings at altitudes of 420-1,650 m, which were occupied all throughout the year. Despite the drastic differences in the living conditions for dust mites in these 2 groups, no significant changes in allergen concentration could be detected for Der p 1 and mite group 2 allergens (Der p 1: P=0.832; mite group 2: P=0.672). Only Der f 1 allergens showed less concentrations in samples taken from buildings closed and unheated during the winter (Der f 1: P=0.005) (Fig. 5).
Air temperature in different buildings ranged from 14.03℃ to 24.96℃ (arithmetic mean: 20.55℃±2.7℃). There was a clear negative correlation of temperature to rising altitude (r=-0.537; P<0.001), but no relationship between temperature and the concentration of mite allergens could be measured (Der p 1: r=-0.133, P=0.201, Der f 1: r=0.027, P=0.799, mite group 2: r=-0.077, P=0.459). There was also a high variation in RH, ranging from 21% to 56% (arithmetic mean: 44.05±8.95%), and RH also showed a clear negative correlation with altitude (r=-0.635; P<0.001). However, in contrast to previous studies, there was no correlation between allergen concentrations and RH (Der p 1: r=-0.158; P=0.129; Der f 1: r=-0.047; P=0.652; mite group 2: r=-0.102; P=0.328) (Fig. 6).
Fine dust PM10 ranged from 5.70 to 468.50 µg/m3(arithmetic mean: 57.49±71.90 µg/m3, PM-2.5 from 1.18 to 56.37 µg/m3(arithmetic mean: 11.44±11.20 µg/m3, and PM-1 from 0.39 to 47.28 µg/m3(arithmetic mean: 7.51±8.60 µg/m3. Indoor fine dust PM values (n=111) showed no correlation with altitude or the weight of collected dust samples. A positive correlation was found for the weight of dust in the collected samples from floors and mattresses with concentration of mite allergens (Der p 1: r=0.359, P<0.001; Der f 1: r=0.398, P<0.001; Mite group 2: r=0.406, P<0.001). Concentrations of mite allergens also showed weak negative correlations with most of the measured PM (Table).
Interestingly, indoor environmental characteristics, such as the type of heating, number of windows and beds, type of glazing, application, quantity of users (day and night), subjective quantity of linens used in the room, age of building or type of ventilation, also showed no association with concentrations of mite allergens. Not even methods or frequencies of cleaning were related to levels of detected allergens. A insignificant difference could be found between different types of floors. Stone floors displayed the lowest allergen concentrations (arithmetic mean: Der p 1: 20.93 ng/g, Der f 1: 11.44 ng/g, mite group 2: 10.86 ng/g), whereas vinyl floors showed the highest concentrations of Der p 1 and mite group 2 allergens (arithmetic mean Der p 1: 56.12 ng/g, mite group 2: 29.30 ng/g). The highest Der f 1 allergen concentrations were found on laminate floors (arithmetic mean Der f 1: 124.83 ng/g).
The results of the present study, performed in alpine regions of Germany and Austria, clearly indicate that the concentration of the HDM allergens Der p 1 and mite group 2 does not significantly decrease at high altitude. Moreover, no correlation between altitude, RH, and HDM allergen concentrations could be found. Even in mountain huts at high altitude that were not used in winter and thus were fully frozen at that time, HDM concentrations did not differ from buildings located at lower altitudes which were occupied throughout the year. Similarly, dwelling conditions did not significantly alter the allergen load. Only Dermatophagoides farinae shows a weak negative but significant correlation with rising altitudes. Based on this fact, we assume a higher sensitivity of the American HDM and poorer ability to adapt to changes. The dry and cold air at high altitude may also affect the stability of Der f 1 allergen in comparison with Der p 1 and mite group 2 allergens.
As one would expect, outdoor air pollution at high altitude is generally lower; however, indoor fine dust pollution did not significantly change with altitude. The difference in allergen concentration in the disturbed air and the settled dust could be attributed to the feature of fine dust, binding to allergens on its surface. The higher the particulate matter, the more allergens are hovering in the disturbed air, compared to allergen levels determined in the settled dust on floors or mattresses. This might have a big impact on the health-related quality of life of HDM allergic patients in rooms with high fine dust pollution.
Our data are in line with publications questioning the paradigm "the higher altitude, the lower the allergen concentrations",24252627 which was primarily based on the assumption that a hostile environment to mites accompanied by a reduction in the number of mites (or elimination of mites during winter in frozen buildings) may eliminate the allergens. This biological view may be correct for the mite itself, but its proteins can be stable over a long period of time. Obviously, this is the case with HDM allergens, and high HDM allergen concentrations measured in chalets at high altitude that are frozen for several months a year favor this interpretation. We postulate, that after mites die away, the allergen concentration remains high for a prolonged period, and freezing may even prolong the amount of intact allergens. It has already been shown that mite allergens are chemically stable for up to four years.44 During warmer periods, small populations of mites regenerate, and as people return, more mites are imported, and they deposit "fresh" allergen again. It would be interesting, especially in these specific chalets or mountain huts, to measure the fluctuation in the mite population and the allergen concentration. Due to the mechanism we postulate, the fluctuation in the latter should be low, and in sum the allergen load would be as high as at lower altitudes.
Nevertheless, there is evidence of a salutary effect that accompanies a stay in high-altitude regions for people allergic to HDM, which has been demonstrated in both pediatric4546 and adult patients with allergic and intrinsic, moderate and severe asthma.47 Interestingly, patients with intrinsic asthma also show a reduction in airway inflammation.
Taken together, it is clearly indicated that the mitigating effect of high-altitude climate therapy for allergic asthma can no longer be ascribed to a reduction in HDM allergen in the environment. Other mechanisms remain to be responsible for the beneficial effects. Recent studies address several factors, such as reduced humidity, low endotoxin load, decreased exposure to fungal spores, pollen, and air pollution, which may contribute to the positive influence of high climate therapy on allergic patients and asthmatics.454748 Furthermore, higher air fluidity and lower oxygen concentration at high altitude reduce respiratory resistance and aid in breathing. In addition, increased UV-light exposure can modulate the immune system and holiday-makers visiting high-altitude regions experience a relief from stress during their stay. Also these parameters may have an influence on allergy symptoms and asthma control, comparable to medicinal immunotherapies.4849
The contradictory data concerning the occurrence of HDM, based on altitude and RH, may be partially explained by changes in building construction (modern insulation, heating, and glazing) and climate changes (global warming) since the 1970s.350 However, our data also contradict the widespread assumption that improved isolation of buildings and more frequent heating of rooms, in comparison to previous decades, leads to a higher allergen concentration at high altitude. Again, the HDM concentration in buildings completely frozen during several winter months provides the strongest argument against this postulate.
Similarly, postulations from older papers have to be critically addressed, e.g. it has been reported that HDMs need a RH of 70%-80% to develop, and stop growing and die at a RH of 60% and lower.1933 We measured RH in most of the rooms in which the 122 dust samples were collected for this study. Though the relative air humidity was below 60% in all rooms (n=53), all the samples contained HDM allergens. Whether humidity is not the only and/or major controlling factor for the development and growth of mite populations, or there is a higher RH of the microenvironment of mites, cannot be determined by this study.
Another reason for the differing results in literature may be improved immuno-chemical techniques, which now allow for the targeted detection of very low allergen concentrations in dust samples.
We provide evidence that HDM allergen levels are not significantly lower in mountainous areas of the alpine region of Germany and Austria. The data indicate that recovery of asthmatics and allergic sufferers during a stay in a high-altitude region is not the result of lacking allergen (allergen avoidance), as has been postulated for several decades. Hitherto unknown mechanisms, possibly triggered by stationary effects or climatic conditions, seem to be responsible for mitigating the allergic immune response, at least its effector phase.
Figures and Tables
Fig. 1
Total number of allergens: Data shown as distribution of individual allergen concentrations of Der p 1, Der f 1, and Mite group 2 with means (grey lines). n=122 each.
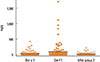
Fig. 2
Percentage of allergen concentrations of Der p 1, Der f 1 and Mite group 2 of the samples taken at 400-1,500 m a.s.l. (n=65) and 1,500-2,600 m a.s.l. (n=57). There are significant higher Der f 1 allergen levels compared to those of Der p 1 (P=0.001) below 1,500 m, but no concentration differences at higher altitude (P=0.689).
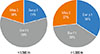
Fig. 3
Allergen concentrations of Der p 1, Der f 1, and Mite group 2 of the samples taken above (n=57) and below 1,500 m a.s.l. (n=65). There are significant lower Der f 1 allergen levels at high altitude compared to allergen levels below 1,500 m a.s.l. (P<0.001). For Der p 1 and Mite 2 allergens, no concentration differences between high and low altitudes could be detected (Der p 1: P=0.928; Mite 2: P=0.127).

Fig. 4
Comparison of allergen concentrations of the samples taken from floors (F) and mattresses (M) with standard deviations. Allergen concentrations of all 3 HDM allergens were significantly higher in mattresses (Der p 1: P=0.001; Der f 1: P=0.005; Mite group 2: P=0.017).

Fig. 5
HDM allergen concentrations in different habitats. Means and standard deviation indicate allergen concentrations given in ng/g of dust of the samples from buildings used year-round (n=85) and from those used only half a year (n=37).

Fig. 6
Relative air humidity (RH) to allergen concentration. The linear regression indicates no correlation between Der p 1, Der f 1, and Mite group 2 allergens with relative air humidity.
ACKNOWLEDGMENTS
We want to thank Hans Leutgeb, deputy mayor of Krimml, Austria, and Wolfgang Taxer for their important input. We also want to thank Petra Lemberger, TVB Krimml and Erich Czerny, HTH for their valuable contributions and discussion of the science.
This work was funded by the EU-Interreg Project Italy-Austria "TRAIL FOR HEALTH - Sviluppo prodotto turistico turismo e salute/TRAIL. FOR HEALTH - Gesundheitstourismus als touristisches Ganz-jahres-produkt".
References
1. Bachert C, Lange B, Virchow JC. Asthma und allergische rhinitis. 1st ed. Stuttgart: Thieme;2005.
2. Seo JH, Kim HY, Jung YH, Lee E, Yang SI, Yu HS, et al. Interactions between innate immunity genes and early-life risk factors in allergic rhinitis. Allergy Asthma Immunol Res. 2015; 7:241–248.
3. Kim BJ, Lee SY, Kim HB, Lee E, Hong SJ. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol Res. 2014; 6:389–400.
4. Gough L, Schulz O, Sewell HF, Shakib F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999; 190:1897–1902.
5. Hammad H, Charbonnier AS, Duez C, Jacquet A, Stewart GA, Tonnel AB, et al. Th2 polarization by Der p 1--pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood. 2001; 98:1135–1141.
6. Traidl-Hoffmann C, Jakob T, Behrendt H. Determinants of allergenicity. J Allergy Clin Immunol. 2009; 123:558–566.
7. Platts-Mills TA. The role of indoor allergens in chronic allergic disease. J Allergy Clin Immunol. 2007; 119:297–302.
8. Woodcock A, Custovic A. ABC of allergies. Avoiding exposure to indoor allergens. BMJ. 1998; 316:1075–1078.
9. De Alba J, Raemdonck K, Dekkak A, Collins M, Wong S, Nials AT, et al. House dust mite induces direct airway inflammation in vivo: implications for future disease therapy? Eur Respir J. 2010; 35:1377–1387.
10. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004; 169:378–385.
11. Khlgatian SV, Perova NA. Allergens from Dermatophagoides dust mites: origin, antigenic and structural characteristics, and therapeutic agents. Biokhimiia. 1995; 60:218–237.
12. Sidenius KE, Hallas TE, Stenderup J, Poulsen LK, Mosbech H. Decay of house-dust mite allergen Der f 1 at indoor climatic conditions. Ann Allergy Asthma Immunol. 2002; 89:34–37.
13. de Boer R, van der Hoeven WA, Stapel SO. The decay of house dust mite allergens, Der p I and Der p II, under natural conditions. Clin Exp Allergy. 1995; 25:765–770.
14. Platts-Mills TA, Heymann PW, Chapman MD, Hayden ML, Wilkins SR. Cross-reacting and species-specific determinants on a major allergen from Dermatophagoides pteronyssinus and D. farinae: development of a radioimmunoassay for antigen P1 equivalent in house dust and dust mite extracts. J Allergy Clin Immunol. 1986; 78:398–407.
15. Grootendorst DC, Dahlén SE, Van Den Bos JW, Duiverman EJ, Veselic-Charvat M, Vrijlandt EJ, et al. Benefits of high altitude allergen avoidance in atopic adolescents with moderate to severe asthma, over and above treatment with high dose inhaled steroids. Clin Exp Allergy. 2001; 31:400–408.
16. Huss K, Adkinson NF Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001; 107:48–54.
17. Gómez MS, Portus M, Gallego J. Factors influencing the house dust mite population. IV. Altitude Allergol. Allergol Immunopathol (Madr). 1981; 9:123–130.
18. Vervloet D, Penaud A, Razzouk H, Senft M, Arnaud A, Boutin C, et al. Altitude and house dust mites. J Allergy Clin Immunol. 1982; 69:290–296.
19. Spieksma FT, Zuidema P, Leupen MJ. High altitude and house-dust mites. BMJ. 1971; 1:82–84.
20. Menz G, Petri E, Lind P, Virchow C. House dust mite in different altitudes of Grisons. Experientia Suppl. 1987; 51:197–201.
21. Charpin D, Birnbaum J, Haddi E, Genard G, Lanteaume A, Toumi M, et al. Altitude and allergy to house-dust mites. A paradigm of the influence of environmental exposure on allergic sensitization. Am Rev Respir Dis. 1991; 143:983–986.
22. de Andrade AD, Bartal M, Birnbaum J, Lanteaume A, Charpin D, Vervloet D. House dust mite allergen content in two areas with large differences in relative humidity. Ann Allergy Asthma Immunol. 1995; 74:314–316.
23. Menz G. Effect of sustained high altitude on asthma patients. Expert Rev Respir Med. 2007; 1:219–225.
24. Gitoho F, Rees P. High altitude and house-dust mites. BMJ. 1971; 3:475.
25. Valdivieso R, Iraola V, Pinto H. Presence of domestic mites at an extremely high altitude (4800 m) in Andean Ecuador. J Investig Allergol Clin Immunol. 2009; 19:323–324.
26. Valdivieso R, Iraola V, Estupiñán M, Fernández-Caldas E. Sensitization and exposure to house dust and storage mites in high-altitude areas of ecuador. Ann Allergy Asthma Immunol. 2006; 97:532–538.
27. Valdivieso R, Estupiñan M, Acosta ME. Asthma and its relation with Dermatophagoides pteronyssinus and Dermatophagoides farinae in Andean altitudes (Quito, Ecuador). J Investig Allergol Clin Immunol. 1997; 7:46–50.
28. Blackhall K, Appleton S, Cates CJ. Ionisers for chronic asthma. Cochrane Database Syst Rev. 2003; (3):CD002986.
29. Peroni DG, Boner AL, Vallone G, Antolini I, Warner JO. Effective allergen avoidance at high altitude reduces allergen-induced bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1994; 149:1442–1446.
30. Peroni DG, Piacentini GL, Costella S, Pietrobelli A, Bodini A, Loiacono A, et al. Mite avoidance can reduce air trapping and airway inflammation in allergic asthmatic children. Clin Exp Allergy. 2002; 32:850–855.
31. Valletta EA, Piacentini GL, Del Col G, Boner AL. FEF25-75 as a marker of airway obstruction in asthmatic children during reduced mite exposure at high altitude. J Asthma. 1997; 34:127–131.
32. van Velzen E, van den Bos JW, Benckhuijsen JA, van Essel T, de Bruijn R, Aalbers R. Effect of allergen avoidance at high altitude on direct and indirect bronchial hyperresponsiveness and markers of inflammation in children with allergic asthma. Thorax. 1996; 51:582–584.
33. Charpin D, Kleisbauer JP, Lanteaume A, Razzouk H, Vervloet D, Toumi M, et al. Asthma and allergy to house-dust mites in populations living in high altitudes. Chest. 1988; 93:758–761.
34. Eggleston PA. Improving indoor environments: reducing allergen exposures. J Allergy Clin Immunol. 2005; 116:122–126.
35. Arlian LG, Neal JS, Morgan MS, Vyszenski-Moher DL, Rapp CM, Alexander AK. Reducing relative humidity is a practical way to control dust mites and their allergens in homes in temperate climates. J Allergy Clin Immunol. 2001; 107:99–104.
36. Dornelas de Andrade A, Birnbaum J, Lanteaume A, Izard JL, Corget P, Artillan MF, et al. Housing and house-dust mites. Allergy. 1995; 50:142–146.
37. Cho SH, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, et al. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci Total Environ. 2006; 371:31–43.
38. van Strien RT, Gehring U, Belanger K, Triche E, Gent J, Bracken MB, et al. The influence of air conditioning, humidity, temperature and other household characteristics on mite allergen concentrations in the northeastern United States. Allergy. 2004; 59:645–652.
39. McCormack MC, Breysse PN, Matsui EC, Hansel NN, Peng RD, Curtin-Brosnan J, et al. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann Allergy Asthma Immunol. 2011; 106:308–315.
40. Jacquemin B, Schikowski T, Carsin AE, Hansell A, Krämer U, Sunyer J, et al. The role of air pollution in adult-onset asthma: a review of the current evidence. Semin Respir Crit Care Med. 2012; 33:606–619.
41. Vernon MK, Wiklund I, Bell JA, Dale P, Chapman KR. What do we know about asthma triggers? A review of the literature. J Asthma. 2012; 49:991–998.
42. Sun JL, Shen L, Chen J, Yu JM, Yin J. Mite and booklouse fauna from vacuumed dust samples from beijing. Allergy Asthma Immunol Res. 2014; 6:257–262.
43. King EM, Filep S, Smith B, Platts-Mills T, Hamilton RG, Schmechel D, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J Immunol Methods. 2013; 387:89–95.
44. Richardson G, Eick S, Jones R. How is the indoor environment related to asthma?: literature review. J Adv Nurs. 2005; 52:328–339.
45. Charpin D. High altitude and asthma: beyond house dust mites. Eur Respir J. 2012; 40:1320–1321.
46. Vervloet D, Bongrand P, Arnaud A, Boutin C, Charpin J. Objective immunological and clinical data observed during an altitude cure at Briançon in asthmatic children allergic to house dust and dermatophagoides (author's transl). Rev Fr Mal Respir. 1979; 7:19–27.
47. Karagiannidis C, Hense G, Rueckert B, Mantel PY, Ichters B, Blaser K, et al. High-altitude climate therapy reduces local airway inflammation and modulates lymphocyte activation. Scand J Immunol. 2006; 63:304–310.
48. Rijssenbeek-Nouwens LH, Fieten KB, Bron AO, Hashimoto S, Bel EH, Weersink EJ. High-altitude treatment in atopic and nonatopic patients with severe asthma. Eur Respir J. 2012; 40:1374–1380.
49. Kim SH, Mun SJ, Han DH, Kim JW, Kim DY, Rhee CS. Three-year follow-up results of sublingual immunotherapy in patients with allergic rhinitis sensitized to house dust mites. Allergy Asthma Immunol Res. 2015; 7:118–123.
50. Bielory L, Lyons K, Goldberg R. Climate change and allergic disease. Curr Allergy Asthma Rep. 2012; 12:485–494.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download