Abstract
In susceptible individuals, inhalation of Aspergillus spores can affect the respiratory tract in many ways. These spores get trapped in the viscid sputum of asthmatic subjects which triggers a cascade of inflammatory reactions that can result in Aspergillus-induced asthma, allergic bronchopulmonary aspergillosis (ABPA), and allergic Aspergillus sinusitis (AAS). An immunologically mediated disease, ABPA, occurs predominantly in patients with asthma and cystic fibrosis (CF). A set of criteria, which is still evolving, is required for diagnosis. Imaging plays a compelling role in the diagnosis and monitoring of the disease. Demonstration of central bronchiectasis with normal tapering bronchi is still considered pathognomonic in patients without CF. Elevated serum IgE levels and Aspergillus-specific IgE and/or IgG are also vital for the diagnosis. Mucoid impaction occurring in the paranasal sinuses results in AAS, which also requires a set of diagnostic criteria. Demonstration of fungal elements in sinus material is the hallmark of AAS. In spite of similar histopathologic features, co-existence of ABPA and AAS is still uncommon. Oral corticosteroids continue to be the mainstay of management of allergic aspergillosis. Antifungal agents play an adjunctive role in ABPA as they help reduce the fungal load. Saprophytic colonization in cavitary ABPA may lead to aspergilloma formation, which could increase the severity of the disease. The presence of ABPA, AAS, and aspergilloma in the same patient has also been documented. All patients with Aspergillus-sensitized asthma must be screened for ABPA, and AAS should always be looked for.
Aspergillosis of the respiratory tract has diverse manifestations that range from hypersensitivity disorders to rapidly invasive disseminated disease.12 These can be classified into 3 distinct clinical categories, viz. allergic aspergillosis, saprophytic colonization, and invasive aspergillosis (Table 1). Different presentations of the allergic form, usually seen in atopic individuals, include Aspergillus-induced asthma (AIA), allergic bronchopulmonary aspergillosis (ABPA), and allergic Aspergillus sinusitis (AAS). This review focuses on ABPA and highlights some of the other Aspergillus-related respiratory disorders.
Patients with asthma who have a positive immediate (type I) IgE-mediated hypersensitivity to Aspergillus are grouped as AIA. A wide variation to the tune of 16% to 38% has been observed in Aspergillus sensitization among asthmatics across the world.345 One of the initial studies found a then considered "unexpected" finding of more severe airway obstruction in patients with AIA.3 In our study of 105 patients with asthma, positive skin reactivity to Aspergillus antigens was noted in 30 subjects (28.5%).5 The disease was more severe in these patients with AIA as evidenced by a statistically significant higher mean duration of illness (P<0.001), mean eosinophil count (P<0.0001), mean total IgE (P<0.05), and more usage of oral corticosteroids per year (P<0.004).
The term 'severe asthma with fungal sensitization' (SAFS) was coined for a subset of asthmatics that demonstrated sensitization to fungal antigens and had frequent exacerbations of asthma that necessitated admission to the hospital.6 Diagnostic criteria for SAFS include: (1) severe (poorly controlled) asthma, (2) either a positive skin prick test result for fungi (but not necessarily to Aspergillus species) or in vitro demonstration of antifungal IgE of at least 0.4 kU/L, (3) total serum IgE concentration <1,000 kU/L.6 Unlike in ABPA, patients with SAFS do not have mucoid impaction or bronchiectasis. Another important difference between SAFS and ABPA is that while severe asthma is one of the diagnostic criteria for SAFS, ABPA also develops in those with mild or moderate asthma. Greenberger7 has also highlighted the divergence between SAFS and ABPA.
Given the persistent fungal colonization of the bronchi in patients with allergic aspergillosis, a potential role for antifungal therapy has been suggested.8 Significantly better asthma quality of life scores were found when itraconazole was administered to patients with SAFS.9 However, a recent study using voriconazole for 3 months in moderate-to-severe asthmatics sensitized to Aspergillus fumigatus (Af) did not show any benefit.10 The authors suggested that the beneficial effects of itraconazole in previous studies could possibly be due to its pharmacokinetic effects on corticosteroids.
ABPA is the most significant manifestation of allergic aspergillosis that occurs worldwide but has not received the importance that it deserves.11 Most commonly seen in patients with asthma and cystic fibrosis (CF), ABPA is caused by hypersensitivity to Aspergillus antigens. In susceptible hosts, an allergic response is evoked by repeated inhalation of Aspergillus spores. The fungal antigens, chiefly of Af, elicit mainly a type I (IgE-mediated) reaction that is responsible for the disease presentation. Type-III (IgG-mediated immune complex) and Type-IV (cell mediated) responses have also been implicated, but tissue invasion does not occur.12 When fungi other than Aspergillus are responsible for such a condition, it is termed as allergic bronchopulmonary mycoses (ABPM).1314 Based on specific pathophysiological mechanisms, it has been proposed that ABPA/ABPM be classified as a distinct endotype of asthma.15
Although 63 years have passed since this disease was first described in England,16 we are still unable to fathom the reason why only a few patients with asthma are affected with ABPA. Individual host genetic susceptibility appears more significant than environmental factors in the causation of ABPA in these subjects. Moreover, although familial preponderance is very common in asthma, occurrence of ABPA among family members is rare.1718 We have detected familial occurrence in 4 pairs (4.9%) of first-degree relatives. One patient each in 3 of these 4 pairs also had concomitant AAS.19
The exact prevalence of this disease is still not known, and this is most likely due to the lack of a uniform diagnostic criterion and standardized tests.20 This potentially destructive lung disease is yet to be included in the ninth revision of the International Classification of Diseases published in 1996.21 Prior to 1968, when ABPA was unknown outside Europe, the prevalence of definite ABPA among asthmatics was around 8%-11%, while that of probable ABPA was approximately 22%.22 After the first report from the United States in 1968,23 awareness regarding ABPA grew across all continents. Between 1983 and 1986, Greenberger and Patterson24 from the United States found ABPA in 32 (6%) out of 531 asthmatic patients having immediate cutaneous reactivity to Aspergillus antigens. In other studies, ABPA was detected in as many as 25% to 37% of asthmatics with a positive skin prick test to Af.25 Among 105 patients with bronchial asthma, we noted a significantly longer duration of illness, earlier age of onset of asthma as well as rhinitis, higher mean total leucocyte counts, absolute eosinophil counts, and total serum IgE values in 8 patients diagnosed with ABPA when compared to those with Aspergillus sensitization only without ABPA.5
Western estimates suggest that ABPA complicates up to 6% of all chronic cases of asthma.26 The prevalence of ABPA in patients with underlying CF ranges from 2% to 15%.27 Denning et al.28 in a scoping review based on the published studies on asthma and ABPA, attempted to ascertain the global burden of ABPA. The prevalence of ABPA in adult asthmatics, as analyzed from 5 prospective studies having at least 50 patients with asthma, was found to be 2.5% (range 0.72%-3.5%). Based on this, the authors deduced that adult patients with ABPA across the globe could "potentially exceed 4.8 million."28
Since there were no consensus-based guidelines on ABPA so far, the International Society for Human and Animal Mycology (ISHAM), in September 2011, constituted a Working Group on ABPA complicating asthma.29 Data on Aspergillus sensitization and ABPA published since circa 2000 was collected by the ISHAM Working Group.29 The prevalence of Aspergillus sensitization among patients with asthma ranged from 5.5%-38.5%, and the prevalence of ABPA in asthma varied between 2.5% and 22.3% with a pooled prevalence of 8.4%.
Immune mediated mechanisms of lung destruction in ABPA are not fully understood. Af antigens elicits a polyclonal antibody response which is largely responsible for elevated levels of total IgE as well as Af-IgE and Af-IgG antibodies.3031 Increased interleukin (IL)-4, IL-5, IL-10, and IL-13 production due to the cellular Th-2 immunologic response suggests an immunocompetent host.3233 We have identified antibodies to a cytotoxic ribonuclease antigen (18 kD) and an elastinolytic protease antigen (45kD) in Indian patients with ABPA.3435 Genetic risk factors include expression of HLA-DR2 and HLA-DR5 genotypes, while HLA-DQ2 protected against ABPA.3637 In subjects with CF, increased chances of Aspergillus colonization of the airways and subsequent development of ABPA were found in those with CF transmembrane conductance regulator gene mutations.383940 Surfactant protein-A2 polymorphisms,41 elevated levels of mannan-binding lectin due to the 1011A allele,42 and toll-like receptor polymorphisms43 also play an important role in the development of ABPA. An immunoproteomics approach would help identify synthetic peptide antigens of Af for skin testing, serodiagnosis, and potentially immunotherapy.4445
As our understanding of the disease is improving, diagnostic criteria continue to evolve. In 1952, Hinson et al.16 reported 3 patients of "a kind not previously recognized". These patients presented with repeated episodes of fever, productive cough, wheezing dyspnoea, and occasional chest pain. Eosinophilia, pulmonary infiltrates in different areas on the chest roentgenogram, and Aspergillus mycelium on microscopic examination of the purulent sputum were found during acute attacks. Saccular bronchiectasis was noted in 2 cases. Due to certain peculiar features not usually observed in patients with pulmonary eosinophilia, the authors suggested to classify their 3 patients as a separate entity. Unlike the acute presentation of pulmonary eosinophilia, these patients had a protracted course of illness. It was also observed that production of sputum "plugs" correlated with clearing of radiologic infiltrates. Mucosal edema and bronchial spasm without any obstructing masses were found on bronchoscopy, while markedly dilated bronchi filled with sticky, tenacious mucus were necropsy findings in 1 patient. The authors stated that, "....intense eosinophilic infiltration and excessive production of mucus represented an allergic response...." Since fungal masses were not observed, they did not group these patients under "mycetomata."
Once the disease was recognized in the United States in 1968 and thence globally, the key diagnostic features have been standardized.4647 Based on clinical, radiologic, and laboratory features, a set of 8 major and 3 minor criteria was proposed in 1977 by Rosenberg and Patterson,46 which remains the most well-acknowledged criteria (Table 2). Although a set of criteria is required, there is no single test that establishes the diagnosis other than demonstration of central bronchiectasis (CB) with normal tapering bronchi, a feature still considered pathognomonic of ABPA.4849 However, CB has also been found to extend to the periphery in some segments.50
All the 8 major criteria may not be found at all times. Some of the features may be present only during the acute (stage 1) or the exacerbation (stage 3) states. Moreover, apart from CB and Aspergillus type-1 hypersensitivity, the other parameters are affected by therapy with prednisolone. This makes it difficult for all criteria to always be fulfilled in patients with ABPA. In 2002, Greenberger51 advocated a set of minimally essential criteria, which includes (1) asthma, (2) immediate cutaneous reactivity to Af, (3) total serum IgE >1,000 ng/mL (417 kU/L), (4) elevated specific IgE-Af/IgG-Af, and (5) CB in the absence of distal bronchiectasis. Greenberger7 in 2013 further proposed "truly minimal" diagnostic criteria that included items (1), (2), (3), and (5) of the aforementioned minimally essential criteria.
Central or proximal bronchiectasis with normal peripheral bronchi continues to be considered a sine qua non for the diagnosis of ABPA.48 However, there exists a subset of patients with a milder form of the disease in whom CB may not be present. These serologically positive patients satisfy the remaining criteria for ABPA and are categorised as ABPA-S.52 Specific treatment for ABPA should immediately be commenced so as to delay or prevent further lung damage. Later on when CB develops, the patient is classified as ABPA-CB.53
Even today, there is no agreement on the minimum number of criteria, either major or minor, required to diagnose ABPA.54 The ISHAM Working Group29 has proposed a set of revised criteria wherein the items are broadly divided into 'obligatory' and 'other' criteria (Table 2). Bronchial asthma and CF are identified as predisposing conditions for ABPA in this newly proposed set of criteria. The 2 features of the obligatory criteria are as follows: (1) positive immediate (type I) cutaneous hypersensitivity to Aspergillus antigen or elevated IgE levels against Af and (2) elevated total IgE levels >1,000 IU/mL. Both of these findings must be present to establish a diagnosis of ABPA. At least 2 out of 3 other criteria viz. (1) presence of precipitating or IgG antibodies against Af in serum, (2) radiographic pulmonary opacities consistent with ABPA, and (3) total eosinophil count >500 cells/µL in steroid naïve patients should be fulfilled. However, the Working Group29 has suggested that this newly proposed criteria needs "validation and further refinement."
Based on the Epidemiologic Study of Cystic Fibrosis (ESCF) database,55 a set of criteria for the diagnosis of acute ABPA in patients with CF has been laid down. The ESCF criteria adopted included the presence of 2 of the following 3: (1) immediate skin reactivity to Af antigens, (2) precipitating antibodies to Af antigens, and (3) total serum IgE >1,000 IU/mL; and at least 2 of the following 6: (1) bronchoconstriction, (2) peripheral blood eosinophilia >1,000/µL, (3) history of pulmonary infiltrates, (4) elevated specific IgE-Af/IgG-Af, (5) Af in sputum by smear or culture, and (6) response to steroids.
Although ABPA is predominantly a disease of asthmatics, this entity has also been diagnosed in patients without asthma. After the first such description in 1981,56 more than a score of patients have been documented. A noteworthy aspect of this subset of patients was that more than half were initially worked up for bronchogenic carcinoma. Furthermore, the remarkable radiologic similarity to pulmonary tuberculosis has important clinical implications in high tuberculous prevalent areas, as the patient reported by us was referred as 'multidrug-resistant tuberculosis' for evaluation.57 The presence of broncholithiasis in ABPA without asthma has also been described.58
This immunologically mediated lung disease is usually indolent in nature and has a protracted course of illness. The presentation can range from mild asthma, with very few symptoms, to extensive lung disease that may manifest as respiratory failure. Patients encounter repeated episodes of acute exacerbations that, after treatment, are followed by periods of remissions. If left untreated, it more often than not results in a chronic fibrotic lung disease that mimics post tubercular fibrotic sequelae.59 Apart from asthma, ABPA may also be associated with other clinical allergic diseases. Although these atopic conditions may manifest at an early age, ABPA is usually seen in the 20s or 30s, but has also been reported in children6061 and even in infants.62 In a patient with poorly controlled asthma and peripheral eosinophilia, expectoration of golden-brown plugs in the sputum should raise the possibility of ABPA.63 A third of the patients, in spite of extensive radiological lesions, may have few or no symptoms at all.64 Hence, it appears that the severity or chronicity of the disease does not correlate with symptomatology.
We reviewed the clinical profile of 113 patients with ABPA, 70 of whom were males.65 The mean age was 32 years, while the mean age of onset of asthma was 21 years. Respiratory symptoms included cough (99%), breathlessness (99%), expectoration (98%), wheezing (97%), and haemoptysis (41%). Nasal symptoms suggestive of upper airways allergy were present in 45%. Expectoration of sputum plugs was reported by 37% of the patients and nasal plugs by 6%. Approximately half of the patients had a personal/family history of atopy. A study from Korea highlighted that ABPA could possibly occur in patients with destructive lung disease due to tuberculosis.66 Physical examination in ABPA may not be fruitful if the patient is asymptomatic. Rhonchi, crepitations, and bronchial breathing may be heard depending on the degree of the lung disease present. Persistent crackles, which do not clear after either a tussive effort or corticosteroid therapy, suggest extensive fibrosis. These patients may also exhibit cyanosis, digital clubbing, and features of cor pulmonale. Associated hypertrophic osteoarthropathy has also been reported.67
Ever since its first description, different radiologic modalities have played an integral part not only in diagnosing ABPA but also in monitoring the progress of the disease.5068 Various imaging techniques employed over a period of time include plain chest roentgenography, bronchography, and computed tomography (CT).
The wide spectrum of plain chest radiographic appearances, transient or permanent in nature (Table 3), is responsible for the 'picturesque' nature of ABPA.686970 The transient opacities were first recognized by Hinson et al.16 in their seminal description of ABPA in 1952 wherein they stated, "...serial radiographs are essential to show the sequence of incidents of lobar or segmental collapse and consolidation, first in one part, then in another and in either lung." Such 'fleeting shadows' were subsequently encountered in most patients with ABPA and were incorporated as 1 of the 8 major criteria enunciated by Rosenberg and Patterson.46 While evaluating 1,340 chest roentgenograms in 113 patients with ABPA, fleeting shadows were documented in 89%.65 These transient pulmonary infiltrates (Figs. 1 and 2) reflect disease activity and are usually observed in either the acute or the exacerbation stage. Mucoid impaction due to secretions in the damaged bronchi, which may clear with or without therapy, is responsible for the transient nature of these pulmonary infiltrates. No area of the lung remains unaffected, but the upper lobes are predominantly involved.50 While these radiological opacities may be easily mistaken for pulmonary tuberculosis in countries with high tuberculosis prevalence, fleeting shadows can be observed on evaluation of serial radiographs of the patient.71 'Recurrent fixed shadows' may ensue when pulmonary infiltrates reappear at the same sites.72
The most commonly observed transient pattern is consolidation or non-homogeneous opacities, seen in up to 91% of patients with ABPA.656970 These are produced as a result of parenchymal infiltration by inflammatory cells, especially eosinophils. The consolidation often clears after therapy, or sometimes even spontaneously, and is not specific to ABPA. Perihilar or 'pseudohilar' infiltrates, found in 40%-77% of patients,6570 are seen surrounding the dilated, central bronchi that are filled with secretions. Such opacities may simulate hilar lymphadenopathy. However, true hilar adenopathy that resolved after therapy has also been reported in ABPA in adults73 as well as in a child.61
Other transient radiologic features, viz. 'tramline' sign, 'toothpaste' shadows, 'gloved fingers' opacities, and 'V-Y' shaped or 'wine glass' shadows are also highly suggestive of ABPA. Bronchial wall edema, which is due to thickening but without any increase in diameter, gives rise to the 'tramline' sign. This feature, observed in 45%-92% of patients,6970 is not specific for ABPA as it is also visualized in patients with asthma, CF, and acute left heart failure. 'Toothpaste' or bandlike shadows, reported in 24%-65% of patients,656970 are formed due to mucoid impaction and retention of respiratory secretions in the distorted bronchi. 'Gloved fingers' opacities are cast by the expanded and rounded ends of the occluded distal bronchi. Such shadows, seen in 11%-23% of patients with ABPA,656970 may disappear after coughing or with treatment. Mucoid impaction in the bronchi of the upper lobes may lead to 'V-shaped'/'wine glass' shadows. This radiologic feature was observed in 27% of our patients.65 Air-fluid levels due to secretions and debris in the dilated central bronchi, found in up to 20% of patients,6570 are also indicative of ABPA. Collapse, both lobar as well as segmental, are not uncommon in ABPA. Proximal occlusion of the bronchi has caused lobar collapse in 14%-39% of patients.656970 We have earlier documented a patient with concomitant ABPA and AAS presenting as a case of middle lobe syndrome.74 Recently, we reported another patient who presented with middle lobe syndrome.75 Although there was significant symptomatic improvement after 2 weeks, the radiological opacity persisted for 4 months. Subsequently, at 6 months while on therapy with prednisolone, the middle lobe reinflated spontaneously.
The irreversible, fibrotic changes in the bronchial walls and parenchyma lead to various permanent opacities, which tend to persist throughout life even when the patient is in remission. These can be depicted on the chest roentgenogram as parenchymal fibrosis in the form of reticulolinear markings or honeycombing, contracted upper lobes, cavitation and localized emphysema. The most characteristic permanent change, however, is the occurrence of CB with normal peripheral bronchi.76 This continues to be recognized as a hallmark of the disease.
It is believed that bronchiectasis occurs in areas with previous consolidation. On plain chest roentgenograms, this is visualized either as parallel-line opacities, representing widening of the bronchi, or as ring opacities, 1-2 cm in diameter, representing dilated bronchi en face. Parallel-line shadows were observed in 65%-70% of patients with ABPA and ring shadows in 45%-68%.656970 Bronchography, once regarded as the gold standard for the demonstration of bronchiectasis but now considered obsolete, gave a one time complete picture of the whole tracheobronchial tree.77
Currently, CT of the thorax, high resolution in particular, is the modality of choice for the demonstration of bronchiectasis. When compared to bronchography, CT had a sensitivity of 83% and a specificity of 92% in detecting CB in patients with ABPA.77 In children with severe asthma, CT scans helped rapidly and safely establish the diagnosis of ABPA.78 Bronchiectasis on CT is characterised by the 'string of pearl' and 'signet ring' appearances (Fig. 3). Although demonstration of CB with normal peripheral bronchi should continue to be regarded as a sine qua non for the diagnosis of ABPA in patients without CF, extension of CB to the periphery was found in 30% of the lobes and 21% of the segments.50
Besides bronchiectasis, the other bronchial abnormalities observed on CT include dilated and totally occluded bronchi, air-fluid levels within dilated bronchi, and bronchial wall thickening. Common parenchymal abnormalities are nonhomogeneous patchy consolidation and parenchymal scarring of varying extent, segmental or lobar collapse, cavities and emphysematous bullae.68 Cavitary lung disease associated with fibrosis in ABPA can often be difficult to differentiate from fibrocavitary lung disease of tuberculous origin.606779
On high-resolution CT, high-attenuation mucous (HAM) plugs (Fig. 4) were reported in 28% of patients with ABPA.80 The ISHAM Working Group29 has highlighted this finding and considers HAM a pathognomonic feature of ABPA. In an analysis of 155 patients with ABPA, the presence of HAM was associated with significantly higher levels of eosinophils, total IgE, and IgE-Af at the time of diagnosis.81 Pleural abnormalities have also been observed in up to 43% of patients with ABPA.50 Pleural effusion, most likely attributable to the mechanical effect of lung collapse, was first documented in 1981 in 2 patients with ABPA.82 We have also reported an ipsilateral pleural effusion secondary to lung collapse, which subsequently cleared on re-expansion of the lobe after steroid therapy, in a patient with ABPA, AAS and an operated aspergilloma.83 Spontaneous pneumothorax, bronchopleural fistula, pleural thickening, and pleural fibrosis have also been described.6872
Apart from radiologic investigations, the laboratory findings useful for diagnosing and monitoring ABPA include skin testing with Aspergillus antigens, peripheral eosinophil count, serum total IgE, Af-specific IgE and IgG, and precipitating antibodies against Af. Expectoration of golden brownish sputum plugs, one of the 3 minor criteria laid down by Rosenberg and Patterson,46 often provides the first clue in patients with asthma and CF. A positive sputum culture for Aspergillus species, another of the minor criteria, was noted in about 58% cases.63
Peripheral blood eosinophilia, one of the major criteria, can often be demonstrated. During exacerbations, most patients have an absolute eosinophil count between 1,000 and 3,000 per cumm. However, eosinophilia is also found in many other lung diseases; while a normal eosinophil count may be seen in patients on treatment with corticosteroids. Since this test is not very specific, the ISHAM Working Group29 has included this test under 'other' criteria. Sputum eosinophilia may be demonstrated in patients with productive cough.
Both type I (immediate) and type III (delayed) skin sensitivity with different Aspergillus antigens can be found in patients with ABPA. While the type III response is completely suppressed by steroid therapy, there is little or no effect on the type I reaction. Both intradermal and prick tests have been used by different researchers to diagnose ABPA. Depending on the geographical area and the manufacturing technique employed, the Aspergillus antigen extracts available are not uniform.84 Currently, the prick test is used for the initial screening of ABPA. If the prick test is negative, then intradermal testing, which is more sensitive than the prick method, can be performed to elicit Aspergillus sensitization. However, higher false positive results are observed with intradermal tests.
Immediate skin hypersensitivity is not highly specific for ABPA. This is evidenced by the fact that approximately up to 40% of all asthmatics29 and up to 56% of patients with CF85 are sensitized to Af. To overcome this, recombinant Af allergens have now been cloned, purified and standardized for testing. In one of the initial studies in Af sensitized patients with CF, 3 out of 6 patients with ABPA did not demonstrate hypersensitivity with recombinant Af allergen I/a (rAsp f I/a).86 When skin testing with rAsp f 1, rAsp f 3, rAsp f 4 and rAsp f 6 was performed on 50 patients with CF (12 with ABPA, 17 with Aspergillus sensitization without ABPA, and 21 not sensitized to Af), no reactivity to 1:100 or higher dilutions of the cloned rAsp f 4 and rAsp f 6 was found in any of the 38 patients without ABPA.87 The authors suggested that these 2 recombinant allergens are reliable markers for ABPA in CF.
Elevated total serum IgE is one of the minimal essential criteria51 as well as a component of the "truly minimal" diagnostic criteria7 both proposed by Greenberger. In spite of being recognized as a key criterion for diagnosing ABPA, there still remains a disagreement among different research groups in the cutoff level for IgE. In the Rosenberg-Patterson criteria,46 the IgE level for diagnosing ABPA was greater than 1,000 IU/mL (-2,500 ng/mL). However, in the minimal essential criteria, Greenberger51 has given a reduced serum IgE level (>417 IU/mL or 1,000 ng/mL) in order to establish the diagnosis. According to the 'ABPA in CF' consensus criteria, serum IgE >500 IU/mL is considered diagnostic.27 The ISHAM Working Group29 proposed a cutoff level of 1,000 IU/mL as was initially set forth by Rosenberg and Patterson.46 This was so because the Working Group29 "felt that a cutoff of 500 IU/mL may lead to overdiagnosis of ABPA." The cutoff IgE value needs to be validated across all populations as it could possibly be affected by both ethnicity and risk of exposure to Aspergillus antigens. In an electronic survey conducted by Greenberger et al.54 among members of the American Academy of Allergy, Asthma, and Immunology (AAAAI), information was sought on the diagnostic criteria and management practices adopted for ABPA. The authors observed that 44.9% of allergists/immunologists used total IgE concentration ≥417 IU/mL when diagnosing ABPA while 42% respondents regarded IgE levels ≥1,000 IU/mL as the cutoff.
Elevated IgE-Af and IgG-Af is also one of the minimal essential criteria51 for the diagnosis of ABPA. Generally, double the serum values of IgE-Af and IgG-Af are found in patients with ABPA as compared to AIA.47 If the controls' values are not available for comparison, then very high serum levels of IgE-Af or IgG-Af, in an appropriate clinical setting, may be diagnostic of ABPA. The ISHAM Working Group29 has suggested IgE-Af level >0.35 kUA/L to be diagnostic.
The radioimmunoassay (RIA) method replaced the previously used enzyme-linked immunosorbent assay (ELISA) for estimating IgE-Af and IgG-Af as low levels of IgE-Af could not be detected by ELISA. However, the RIA technique was limited by the short shelf life of the radioisotope and exposure to radioactivity. Subsequently, the biotin-avidin-linked immunosorbent assay method was employed in 13 patients with ABPA, 9 with AIA, 12 with aspergilloma, and 9 controls without asthma, which resulted in significantly higher IgE-Af levels in patients with ABPA, even at very high dilutions of 1:1,000.88 The authors attributed this finding to a polyclonal antibody response to Aspergillus antigens in patients with ABPA but not in those with AIA. Similar to demonstration of skin hypersensitivity, ABPA can be distinguished from AIA with high specificity (100%) and sensitivity (90%) by using recombinant Af allergens.89 High levels of specific IgE to recombinant Af allergens also helped detect ABPA in patients with underlying CF.90
By the double immunodiffusion technique of Outcherlony, precipitating antibodies against Af could be detected in the unconcentrated serum from 70% of patients.63 Using concentrated serum, this detection rate improved to 92% of patients with a radiological infiltrate.63 These precipitating antibodies have also been found in 10% of asthmatics without ABPA,4 aspergilloma and in different forms of chronic pulmonary aspergillosis (CPA). Denning et al.91 have correlated the presence of complicating features like fibrosis and cavitation with high titres of serum precipitins in patients with ABPA.
Pulmonary function testing does not help confirm the diagnosis of ABPA. In addition to airflow obstruction, a restrictive pattern with reduction in total lung capacity (TLC), vital capacity (VC), forced expiratory volume in the first second (FEV1) and reduced diffusion capacity for carbon monoxide (DLCO) may be observed when the patient presents in the acute or the exacerbation stage.9293 Normalization of some of these parameters may be noticed after treatment with corticosteroids and also during remission. A significant (P<0.05) reduction in FEV1, FEV1/VC ratio and FEF25-75 was observed in patients with ABPA having mean duration of illness greater than 10 years when compared to those with symptoms less than 10 years' duration.93
Five stages of ABPA9495 have been identified, viz. (i) acute, (ii) remission, (iii) exacerbation, (iv) corticosteroid dependent asthma, and (v) fibrotic lung disease (Table 4). Staging of the disease must be done when establishing the diagnosis and should be reassessed during follow-up visits whenever the patient improves or deteriorates. Improvement in symptoms, resolution of radiologic lesions, as well as a decline in total IgE and blood eosinophilia usually occurs with prednisolone therapy, or at times spontaneously. Exacerbations are entirely asymptomatic in approximately one-third of the cases, and may be detected either by the doubling of remission IgE values or demonstration of extensive radiologic opacities. Although remission for prolonged periods is not common, we reported an exacerbation after prolonged remission in a patient with ABPA and an associated aspergilloma.96 Since therapy with prednisolone may mask the characteristic features of the disease, stage IV ABPA is clinically indistinguishable from corticosteroid dependent asthma without ABPA.97
In order to refine these stages, the ISHAM Working Group29 has proposed a new clinical staging of ABPA in asthma (Table 4). Asymptomatic patients diagnosed with ABPA when routinely investigated as per the criteria were categorized as stage 0. This was done to recognize the disease as early as possible so that commencement of appropriate treatment even before the acute presentation (stage 1) could possibly prevent progression to end stage fibrosis. Stage 2 (response) sets in when there is clinical, radiological and serological improvement. However, this newly proposed staging would require prospective validation.
A newly proposed radiological classification of ABPA that is based on thoracic CT findings has also been tabled by the ISHAM Working Group.29 This new classification has 4 categories that correlate the immunological severity of ABPA with various CT features. As the disease progresses from mild to moderate to severe, the radiological classification is as follows: (1) serological ABPA (ABPA-S), (2) ABPA with bronchiectasis (ABPA-B), (3) ABPA with HAM (ABPA-HAM), and (4) ABPA with chronic pleuropulmonary fibrosis (ABPA-CPF). For inclusion in the ABPA-CPF group, there should be at least 2 other radiologic features, apart from bronchiectasis and HAM, viz. pulmonary fibrosis, parenchymal scarring, fibrocavitary lesions, aspergilloma, and pleural thickening.
As described in the recent AAAAI Committee Report54 on ABPA, the goals of treatment of ABPA are to: (i) control symptoms of asthma or CF, (ii) prevent or treat pulmonary exacerbations of ABPA, (iii) reduce or remit pulmonary inflammation, and (iv) mitigate progression to end-stage fibrotic or cavitary disease. Exclusion of ABPA in family members and identification of any potential environmental source of the incriminated fungus should also be stressed upon when managing a patient with ABPA. No definite prognostic indicators for progression or regression of the disease have been identified. In order to successfully achieve these goals, it is important to treat the disease aggressively during the early stages. Oral corticosteroids continue to remain the cornerstone for the management of ABPA. Appropriately designed clinical trials for the treatment of ABPA are lacking. The role of antifungal drugs so far is at best adjunctive.
Oral corticosteroids, till date, remain the most effective drugs for treating ABPA.98 The dosing schedule and duration of therapy for oral steroids remain poorly defined. For Stages I (acute) and III (exacerbation), the most widely accepted protocol is prednisolone 0.5 mg/kg/day given as a single morning dose for the initial 2 weeks and then switched to an alternate-day dose for the next 6-8 weeks.51 Once the total serum IgE declines by at least 35% and resolution of radiologic infiltrates are noted, prednisolone is further tapered by 2.5 to 5 mg every 2 weeks.99 After discontinuation of prednisolone, if achieved, the patient should be monitored every 6 to 8 weeks to ensure that remission is maintained. If there are any features suggestive of relapse, treatment should be recommenced as early as possible. Patients with stage IV ABPA (steroid-dependent asthma) usually require alternate day therapy with prednisolone 10-40 mg for many years to sustain symptom control. Daily prednisolone along with other interventions for the management of cor pulmonale and arterial hypoxemia are needed for patients with end stage lung disease (stage V).26
To minimize the well-known adverse effects of long term steroid therapy, we assessed the feasibility of a biweekly dosing schedule in 26 patients with ABPA with or without AAS.100 Following an initial dosage of prednisolone 0.5 mg/kg/day for two weeks, patients were alternately prescribed either the conventional alternate-day regimen or a twice weekly dosing protocol was adopted. Patients receiving the biweekly regimen also showed a significant improvement in FEV1, total IgE levels, and eosinophil counts. Pulse therapy with intravenous methylprednisolone using 10 to 20 mg/kg/day for 3 consecutive days has been shown to be useful in managing severe and sometimes life-threatening exacerbations among children with ABPA and CF.101 In conjunction with antifungal agents, this could be considered in patients not improving with oral steroid therapy. Inhaled corticosteroids alone help achieve asthma control, but neither do they prevent symptomatic exacerbations of ABPA nor delay progression of lung damage.
The exact role of antifungal agents in the treatment of ABPA is still debated. By reducing the fungal load, antifungal agents help control the antigenic stimulus and thus decrease the inflammatory response.102 Earlier studies with older antifungal molecules, viz. natamycin, hamycin, amphotericin B, miconazole, clotrimazole and ketoconazole did not show much promise. Subsequently, studies with itraconazole demonstrated a reduction in daily corticosteroid doses without clinical deterioration. The Cochrane Database review103 on azoles for ABPA concluded that itraconazole improved clinical outcomes. The dosage of itraconazole recommended is 200 mg twice daily for 4 to 6 months, which is then tapered over the next 4 to 6 months. By inhibiting steroid metabolism and thereby exacerbating adrenal suppression, itraconazole might lead to cushingoid features when used for very long durations.
To avoid drug resistance and possible clinical failure due to suboptimal therapeutic levels, regular monitoring of itraconazole blood levels is advocated.104 In ABPA, the newer azoles, voriconazole and posaconazole, have improved asthma severity in 70% and 78% of patients respectively.105 However, skin cancer has been associated with long-term voriconazole therapy.106 It is still not known whether itraconazole and other newer azoles would successfully replace oral steroids as first-line therapy for ABPA. The results of a randomized trial on monotherapy of itraconazole vs prednisolone in ABPA (MIPA study) are awaited (clinical trials.gov; NCT01321827).
Omalizumab, a monoclonal antibody against IgE, has also been tried in the management of ABPA. Initial studies in patients with underlying CF have demonstrated a significant clinical improvement with reduction in hospitalisation and exacerbations.107108 Usage of oral corticosteroids in these patients also declined. Similar results have also been documented with omalizumab in patients with ABPA due to underlying asthma.109110 This potential of this drug in decreasing or avoiding oral steroids in patients with stage IV (steroid-dependent) disease should be investigated. Randomized trials with omalizumab and possibly other antibodies to IL-4Ra (dupilumab), IL 5 (mepolizumab), and IL 13 (lebrikizumab) are needed to assess their routine usage in ABPA.
Rarely, ABPA has also recently been recognized in patients with chronic obstructive pulmonary disease.111112 The association of ABPA or an overlap condition that resembles ABPA was described in patients with hyper-IgE syndrome, chronic granulomatous disease, and Kartagener's syndrome.25 In these congenital immunodeficiency neutrophilic conditions, it is essential to distinguish ABPA from invasive aspergillosis as mistreatment with systemic steroids may hasten the invasive process, resulting in increased morbidity.
Although the clinical categories of Aspergillus-associated respiratory disorders usually remain mutually exclusive, similar immunopathologic responses may lead to the coexistence of different forms of respiratory aspergillosis. Concomitant occurrence of ABPA and AAS may not be all that uncommon.187483113 Aspergilloma formation has also been documented in patients with ABPA.6779114 We have twice so far reported concurrent ABPA, AAS, and aspergilloma in a single patient.83115
Just as in ABPA, Aspergillus antigens trigger immunologic reactions in the paranasal sinuses, thereby leading to AAS.116117 Those patients with rhinitis who were sensitized to Aspergillus are possibly at higher risk of developing AAS.118119 Here too, a set of criteria is required to establish the diagnosis of AAS. The key components of the diagnostic criteria for AAS are (1) radiological evidence of sinusitis of 1 or more paranasal sinuses; (2) necrosed amorphous tissue along with edematous polyps infiltrated by eosinophils on histopathological evaluation of material from the sinus; (3) demonstration of fungal elements in nasal discharge or in material obtained at the time of surgery by stain or culture; (4) absence of diabetes, previous or subsequent immunodeficiency disease, and treatment with immunosuppressive drugs; and (5) absence of invasive fungal disease at the time of diagnosis or subsequently. The other features considered include: (1) peripheral blood eosinophilia; (2) type I and type III cutaneous hypersensitivity to Aspergillus; (3) precipitating antibodies to Aspergillus antigens; (4) elevated total and Aspergillus specific IgE levels; and (5) characteristic CT appearances. Features, such as passage of nasal plugs, recurrent nasal polyps, and radiographic evidence of pansinusitis, in patients having an atopic background point toward an allergic fungal phenomenon in the upper airways.113 'Allergic mucin,' which is the characteristic nasal pathologic material comprising eosinophils, Charcot-Leyden crystals, cellular debris, and scattered fungal hyphae, is the hallmark of this disease.116 The characteristic CT finding is the presence of heterogeneous densities with serpiginous areas of increased attenuation (Fig. 5) on noncontrast scans.72 Histopathologic confirmation from the inspissated mucus is a sine qua non for the diagnosis of AAS. The current approach to therapy includes an initial surgical debridement, followed by postoperative oral corticosteroids and supportive therapy.113120
Chronic lung damage in ABPA, especially the presence of cavitating lesions, may provide a favourable milieu for aspergilloma formation.6779 Furthermore, steroid therapy could possibly accelerate the development of fungal balls in patients with cavitary lung disease.114 Vice versa, it has also been seen that a pre-existing aspergilloma, by functioning as a nidus for antigen stimulation in susceptible individuals, may lead to ABPA subsequently.121 We have postulated that the coexistence of an aspergilloma would likely lead to an increase in the severity of underlying ABPA.122
The entity CPA was first highlighted by Denning et al.91 in 2003 when they identified a subset of patients with pre-existing structural lung disease who were chronically affected by Aspergillus but did not have any vascular or tissue invasion by the fungal hyphae. These patients were either immunocompetent or had only pulmonary (localised) immune suppression. On the basis of the radiological patterns, they were categorized as (1) chronic cavitary pulmonary aspergillosis (CCPA), (2) chronic fibrosing pulmonary aspergillosis (CFPA), and (3) chronic necrotizing pulmonary aspergillosis (CNPA). In CCPA, progressive cavitation or extension of a pre-existing cavity was noted; and if left untreated, development of chronic scarring and marked pulmonary fibrosis over time led to CFPA. In the presence of underlying confounding factors like alcoholism, smoking, AIDS, diabetes and corticosteroid treatment that caused mild to moderate immune dysfunction, a necrotizing condition developed, usually after enlargement of a thin-walled cavity. This condition occurred rapidly within weeks or gradually over months, and was labeled as CNPA. The classification of CPA has evolved over the last decade.123124 As these 3 forms are not easy to distinguish clinically, a new simplified classification for CPA has been proposed: (1) simple aspergilloma, (2) CCPA (complex aspergilloma) or slowly progressive CNPA (more than 3 months' duration) and (3) subacute invasive pulmonary aspergillosis or rapidly progressive CNPA of less than 3 months' duration.123
Patients with CPA usually have chronic respiratory and constitutional symptoms for at least 3 months, progressive enlargement and formation of new pulmonary cavities, positive serum precipitins against Aspergillus or isolation of Aspergillus spp. from the cavitary lesion, and elevated inflammatory markers (C-reactive protein, plasma viscosity or erythrocyte sedimentation rate). Diagnostic criteria for CPA also include exclusion of other pulmonary conditions like active tuberculosis and malignancy that mimic the symptoms as well as no obvious conditions suggesting an immunocompromised state.91
When cavitation, fibrosis, pleural thickening and aspergilloma develop in patients with ABPA, CPA ensues. In a study of 126 patients with CPA, ABPA was the primary underlying condition in 15 subjects (11.9%) while SAFS was incriminated in 2 subjects (1.6%).125 A scoping review28 also attempted to estimate the global burden of CPA in patients with ABPA. Applying an annual 15% attrition rate while calculating the period prevalence of CPA over 5 years, the global case burden of CPA complicating ABPA was approximately 10% (range, 7%-20%).28
Sensitization to molds in patients with asthma is known to increase the severity of the disease.126 Patients with asthma, eosinophilia, and history of repeated 'pneumonitis' should be evaluated aggressively for ABPA. This would help avoid diagnostic delay and prevent steady lung damage leading to end-stage fibrosis. In high tuberculosis-prevalent regions, the striking radiological resemblance often results in erroneous treatment with antituberculous drugs. Furthermore, poor access to costly and advanced diagnostic modalities like CT scans and mycoserological tests in low-income countries may hamper establishment of the diagnosis. The occurrence of other Aspergillus-related hypersensitivity respiratory disorders must be sought for in all patients with ABPA.127128
Figures and Tables
Fig. 1
Plain chest roentgenogram showing a left-sided perihilar opacity along with non-homogeneous infiltrates in all zones of both lung fields.
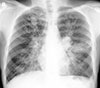
Fig. 2
Plain chest roentgenogram of the same patient taken 4 months later showing spontaneous resolution of the left-sided perihilar opacity. The bilateral non-homogeneous infiltrates have increased considerably. (Figs 1 and 2 reveal 'transient pulmonary infiltrates' or 'fleeting shadows', which are characteristic of ABPA.)
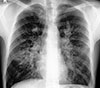
Fig. 3
Computed tomography of the thorax showing 'signet ring' (short, thick arrow) and 'string of pearls' (long, thin arrow) appearances, indicative of central bronchiectasis. Mucoid impaction and dilated bronchi are also visualized.
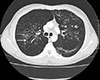
Fig. 4
Computed tomography of the thorax showing 'signet ring' (short, thick arrow) and 'string of pearls' (long, thin arrow) appearances, indicative of central bronchiectasis. Mucoid impaction and dilated bronchi are also visualized.
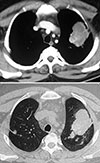
Fig. 5
Computed tomography of the paranasal sinuses showing hyperdense lesions in the ethmoid and maxillary sinuses bilaterally, suggestive of inspissated secretions.
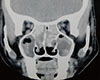
Table 1

Table 2
Evolving diagnostic criteria for ABPA

| Rosenberg-Patterson criteria4647 | Minimal essential criteria51 | 'Truly minimal' criteria7 | ISHAM Working Group29 | ABPA in CF55 |
|---|---|---|---|---|
|
Major criteria 1. Asthma 2. Presence of transient pulmonary infiltrates (fleeting shadows) 3. Immediate cutaneous reactivity to Af 4. Elevated total serum IgE 5. Precipitating antibodies against Af 6. Peripheral blood eosinophilia 7. Elevated serum IgE and IgG to Af 8. Central/proximal bronchiectasis with normal tapering of distal bronchi Minor criteria 1. Expectoration of golden brownish sputum plugs 2. Positive sputum culture for Aspergillus species 3. Late (Arthus-type) skin reactivity to Af |
1. Asthma 2. Immediate cutaneous reactivity to Af 3. Total serum IgE >1,000 ng/mL (417 kU/L) 4. Elevated specific IgE-Af/IgG-Af 5. CB in the absence of distal bronchiectasis |
1. Asthma 2. Immediate cutaneous reactivity to Af 3. Total serum IgE >1,000 ng/mL (417 kU/L) 4. CB in the absence of distal bronchiectasis |
Predisposing conditions 1. Bronchial asthma 2. Cystic fibrosis Obligatory criteria (both should be present) 1. Type I Aspergillus skin test positive (immediate cutaneous hypersensitivity to Aspergillus antigen) or elevated IgE levels against Af 2. Elevated total IgE levels (>1,000 IU/mL)* Other criteria (at least two of three) 1. Presence of precipitating or IgG antibodies against Af in serum 2. Radiographic pulmonary opacities consistent with ABPA 3. Total eosinophil count >500 cells/µL in steroid naïve patients (may be historical) (*If the patient meets all other criteria, an IgE value <1,000 IU/mL may be acceptable) |
Presence of two of the following three: (i) Immediate skin reactivity to Af antigens, (ii) Precipitating antibodies to Af antigens, (iii) Total serum IgE >1,000 IU/mL; and at least two of the following six: (i) Bronchoconstriction, (ii) Peripheral blood eosinophilia >1,000/µL, (iii) History of pulmonary infiltrates, (iv) Elevated specific IgE-Af/IgG-Af, (v) Af in sputum by smear or culture, (vi) Response to steroids |
Table 3

Table 4
Staging in ABPA

| Conventional staging9495 | ||
| Stage I | Acute | The patient is first proved to have ABPA. All the usual features like elevated total as well as Aspergillus-specific IgE, radiological abnormalities, peripheral blood eosinophilia, and serum precipitins against Aspergillus are present. |
| Stage II | Remission | The patient is usually asymptomatic with well controlled underlying asthma. In addition, there should be no new radiological lesions without any rise in total IgE for a period of at least 6 months. |
| Stage III | Exacerbation | There is appearance of fresh pulmonary infiltrates that is often associated with doubling of remission total IgE levels and peripheral blood eosinophilia. |
| Stage IV | Corticosteroid dependent asthma | Patients generally become corticosteroid dependent and oral corticosteroids cannot be tapered off completely. |
| Stage V | Fibrotic lung disease | Radiographic abnormalities in the form of irreversible fibrosis and chronic cavitation persist. Serological parameters are usually negative. |
| ISHAM Working Group proposed clinical staging29 | ||
| Stage 0 | Clinically stable and well controlled asthmatic subjects who do not have any signs and symptoms suggestive of ABPA but are diagnosed as ABPA when routinely investigated as per the criteria | |
| Stage 1 | Acute | Based on CT and/or bronchoscopic findings: |
| 1a: with mucoid impaction | ||
| 1b: without mucoid impaction | ||
| Stage 2 | Response | Clinical as well as radiological improvement associated with at least 25% decline in serum IgE level at 8 weeks of therapy |
| Stage 3 | Exacerbation | Any clinical and/or radiological worsening along with an increase in IgE level by >50% of baseline |
| Stage 4 | Remission | Sustained clinicoradiological improvement accompanied by baseline IgE values (or less than 50% increase) for more than 6 months duration without systemic corticosteroids |
| Stage 5 | 5a: Treatment dependent ABPA | Either relapse occurs on two or more consecutive occasions within 6 months of stopping treatment or there is worsening of clinical, radiological or immunological parameters on tapering oral steroids/azoles |
| 5b: Glucocorticoid dependent asthma | When systemic steroids are required for control of asthma whilst activity of ABPA is under control | |
| Stage 6 | Advanced ABPA | Clinical signs of cor pulmonale and type-2 respiratory failure along with radiological features of fibrosis |
References
1. Shah A. Allergic bronchopulmonary aspergillosis. Indian J Chest Dis Allied Sci. 1998; 40:41–54.
2. deShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997; 337:254–259.
3. Hendrick DJ, Davies RJ, D'Souza MF, Pepys J. An analysis of skin prick test reactions in 656 asthmatic patients. Thorax. 1975; 30:2–8.
4. Schwartz HJ, Citron KM, Chester EH, Kaimal J, Barlow PB, Baum GL, et al. A comparison of the prevalence of sensitization to Aspergillus antigens among asthmatics in Cleveland and London. J Allergy Clin Immunol. 1978; 62:9–14.
5. Maurya V, Gugnani HC, Sarma PU, Madan T, Shah A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest. 2005; 127:1252–1259.
6. Denning DW, O'Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006; 27:615–626.
7. Greenberger PA. When to suspect and work up allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 2013; 111:1–4.
8. Pasqualotto AC, Powell G, Niven R, Denning DW. The effects of antifungal therapy on severe asthma with fungal sensitization and allergic bronchopulmonary aspergillosis. Respirology. 2009; 14:1121–1127.
9. Denning DW, O'Driscoll BR, Powell G, Chew F, Atherton GT, Vyas A, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009; 179:11–18.
10. Agbetile J, Bourne M, Fairs A, Hargadon B, Desai D, Broad C, et al. Effectiveness of voriconazole in the treatment of Aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014; 134:33–39.
11. Shah A, Panjabi C. Allergic bronchopulmonary aspergillosis: a review of a disease with a worldwide distribution. J Asthma. 2002; 39:273–289.
12. Patterson R. Allergic bronchopulmonary aspergillosis and hypersensitivity reactions to fungi. In : Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman's pulmonary diseases and disorders. 3rd ed. New York (NY): McGraw-Hill;1998. p. 777–782.
13. Patterson R. Allergic bronchopulmonary aspergillosis: a historical perspective. Immunol Allergy Clin North Am. 1998; 18:471–478.
14. Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol. 2014; 40:30–48.
15. Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011; 127:355–360.
16. Hinson KF, Moon AJ, Plummer NS. Broncho-pulmonary aspergillosis; a review and a report of eight new cases. Thorax. 1952; 7:317–333.
17. Graves TS, Fink JN, Patterson R, Kurup VP, Scanlon GT. A familial occurrence of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1979; 91:378–382.
18. Shah A, Khan ZU, Chaturvedi S, Malik GB, Randhawa HS. Concomitant allergic Aspergillus sinusitis and allergic bronchopulmonary aspergillosis associated with familial occurrence of allergic bronchopulmonary aspergillosis. Ann Allergy. 1990; 64:507–512.
19. Shah A, Kala J, Sahay S, Panjabi C. Frequency of familial occurrence in 164 patients with allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 2008; 101:363–369.
20. Shah A, Alamoudi O, Al-Mobeireek AF. Allergic bronchopulmonary aspergillosis: a view from India. Saudi Med J. 2002; 23:1559–1560.
21. Novey HS. Epidemiology of allergic bronchopulmonary aspergillosis. Immunol Allergy Clin North Am. 1998; 18:641–653.
22. Henderson AH, English MP, Vecht RJ. Pulmonary aspergillosis. A survey of its occurrence in patients with chronic lung disease and a discussion of the significance of diagnostic tests. Thorax. 1968; 23:513–518.
23. Patterson R, Golbert TM. Hypersensitivity disease of the lung. Univ Mich Med Cent J. 1968; 34:8–11.
24. Greenberger PA, Patterson R. Allergic bronchopulmonary aspergillosis and the evaluation of the patient with asthma. J Allergy Clin Immunol. 1988; 81:646–650.
25. Shah A, Panjabi C. Allergic aspergillosis of the respiratory tract. Eur Respir Rev. 2014; 23:8–29.
26. Greenberger PA. Allergic bronchopulmonary aspergillosis. In : Middleton E, Reed CE, Ellis EF, Franklin AN, Yunginger JW, Busse WW, editors. Allergy: principles and practice. 5th ed. St. Louis (MO): Mosby;1998. p. 981–993.
27. Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003; 37:Suppl 3. S225–S264.
28. Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013; 51:361–370.
29. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013; 43:850–873.
30. Bhatnagar PK, Banerjee B, Shah A, Sarma PU. Probable role of IgG subclasses in patients with allergic bronchopulmonary aspergillosis. Serodiagn Immunother Infect Dis. 1993; 5:123–124.
31. Kurup VP. Immunology of allergic bronchopulmonary aspergillosis. Indian J Chest Dis Allied Sci. 2000; 42:225–237.
32. Wark PA, Gibson PG. Allergic bronchopulmonary aspergillosis: new concepts of pathogenesis and treatment. Respirology. 2001; 6:1–7.
33. Sambatakou H, Pravica V, Hutchinson IV, Denning DW. Cytokine profiling of pulmonary aspergillosis. Int J Immunogenet. 2006; 33:297–302.
34. Madan T, Banerjee B, Bhatnagar PK, Shah A, Sarma PU. Identification of 45 kD antigen in immune complexes of patients of allergic bronchopulmonary aspergillosis. Mol Cell Biochem. 1997; 166:111–116.
35. Purkayastha S, Madan T, Shah A, Krishnamurthy HG, Sarma PU. Multifunctional antigens of A. fumigatus and specific antibodies. Appl Biochem Biotechnol. 2000; 83:271–283.
36. Chauhan B, Santiago L, Kirschmann DA, Hauptfeld V, Knutsen AP, Hutcheson PS, et al. The association of HLA-DR alleles and T cell activation with allergic bronchopulmonary aspergillosis. J Immunol. 1997; 159:4072–4076.
37. Chauhan B, Santiago L, Hutcheson PS, Schwartz HJ, Spitznagel E, Castro M, et al. Evidence for the involvement of two different MHC class II regions in susceptibility or protection in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2000; 106:723–729.
38. Miller PW, Hamosh A, Macek M Jr, Greenberger PA, MacLean J, Walden SM, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in allergic bronchopulmonary aspergillosis. Am J Hum Genet. 1996; 59:45–51.
39. Eaton TE, Weiner Miller P, Garrett JE, Cutting GR. Cystic fibrosis transmembrane conductance regulator gene mutations: do they play a role in the aetiology of allergic bronchopulmonary aspergillosis? Clin Exp Allergy. 2002; 32:756–761.
40. de Almeida MB, Bussamra MH, Rodrigues JC. Allergic bronchopulmonary aspergillosis in paediatric cystic fibrosis patients. Paediatr Respir Rev. 2006; 7:67–72.
41. Saxena S, Madan T, Shah A, Muralidhar K, Sarma PU. Association of polymorphisms in the collagen region of SP-A2 with increased levels of total IgE antibodies and eosinophilia in patients with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2003; 111:1001–1007.
42. Kaur S, Gupta VK, Shah A, Thiel S, Sarma PU, Madan T. Elevated levels of mannan-binding lectin [corrected] (MBL) and eosinophilia in patients of bronchial asthma with allergic rhinitis and allergic bronchopulmonary aspergillosis associate with a novel intronic polymorphism in MBL. Clin Exp Immunol. 2006; 143:414–419.
43. Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis. 2008; 197:618–621.
44. Madan T, Priyadarsiny P, Vaid M, Kamal N, Shah A, Haq W, et al. Use of a synthetic peptide epitope of Asp f 1, a major allergen or antigen of Aspergillus fumigatus, for improved immunodiagnosis of allergic bronchopulmonary aspergillosis. Clin Diagn Lab Immunol. 2004; 11:552–558.
45. Gautam P, Sundaram CS, Madan T, Gade WN, Shah A, Sirdeshmukh R, et al. Identification of novel allergens of Aspergillus fumigatus using immunoproteomics approach. Clin Exp Allergy. 2007; 37:1239–1249.
46. Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977; 86:405–414.
47. Wang JL, Patterson R, Rosenberg M, Roberts M, Cooper BJ. Serum IgE and IgG antibody activity against Aspergillus fumigatus as a diagnostic aid in allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1978; 117:917–927.
48. Scadding JG. The bronchi in allergic aspergillosis. Scand J Respir Dis. 1967; 48:372–377.
49. Shah A. Allergic bronchopulmonary aspergillosis: an emerging disease in India. Indian J Chest Dis Allied Sci. 1994; 36:169–172.
50. Panchal N, Bhagat R, Pant C, Shah A. Allergic bronchopulmonary aspergillosis: the spectrum of computed tomography appearances. Respir Med. 1997; 91:213–219.
51. Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002; 110:685–692.
52. Patterson R, Greenberger PA, Halwig JM, Liotta JL, Roberts M. Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med. 1986; 146:916–918.
53. Greenberger PA, Miller TP, Roberts M, Smith LL. Allergic bronchopulmonary aspergillosis in patients with and without evidence of bronchiectasis. Ann Allergy. 1993; 70:333–338.
54. Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, Knutsen AP. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2014; 2:703–708.
55. Geller DE, Kaplowitz H, Light MJ, Colin AA. Allergic bronchopulmonary aspergillosis in cystic fibrosis: reported prevalence, regional distribution, and patient characteristics. Scientific Advisory Group, Investigators, and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Chest. 1999; 116:639–646.
56. Glancy JJ, Elder JL, McAleer R. Allergic bronchopulmonary fungal disease without clinical asthma. Thorax. 1981; 36:345–349.
57. Shah A, Maurya V, Panjabi C, Khanna P. Allergic bronchopulmonary aspergillosis without clinical asthma caused by Aspergillus niger. Allergy. 2004; 59:236–237.
58. Koh WJ, Han J, Kim TS, Lee KS, Jang HW, Kwon OJ. Allergic bronchopulmonary aspergillosis coupled with broncholithiasis in a non-asthmatic patient. J Korean Med Sci. 2007; 22:365–368.
59. Shah A. Allergic bronchopulmonary aspergillosis: an Indian perspective. Curr Opin Pulm Med. 2007; 13:72–80.
60. Shah A, Bhagat R, Panchal N. Allergic bronchopulmonary aspergillosis with clubbing and cavitation. Indian Pediatr. 1993; 30:248–251.
61. Shah A, Kala J, Sahay S. Allergic bronchopulmonary aspergillosis with hilar adenopathy in a 42-month-old boy. Pediatr Pulmonol. 2007; 42:747–748.
62. Imbeau SA, Cohen M, Reed CE. Allergic bronchopulmonary aspergillosis in infants. Am J Dis Child. 1977; 131:1127–1130.
63. McCarthy DS, Pepys J. Allergic broncho-pulmonary aspergillosis. Clinical immunology: (1) clinical features. Clin Allergy. 1971; 1:261–286.
64. Safirstein BH, D'Souza MF, Simon G, Tai EH, Pepys J. Five-year follow-up of allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1973; 108:450–459.
65. Shah A, Panchal N, Panjabi C. Allergic bronchopulmonary aspergillosis: a review from India [abstract]. Allergy Clin Immunol Int. 2003; Suppl 1. 104.
66. Kim JH, Jin HJ, Nam YH, Hwang EK, Ye YM, Park HS. Clinical features of allergic bronchopulmonary aspergillosis in Korea. Allergy Asthma Immunol Res. 2012; 4:305–308.
67. Shah A, Khan ZU, Chaturvedi S, Ramchandran S, Randhawa HS, Jaggi OP. Allergic bronchopulmonary aspergillosis with coexistent aspergilloma: a long-term followup. J Asthma. 1989; 26:109–115.
68. Shah A, Panchal N, Agarwal AK. Allergic bronchopulmonary aspergillasis: the spectrum of roentgenologic appearances. Indian J Radiol Imaging. 1999; 9:107–112.
69. McCarthy DS, Simon G, Hargreave FE. The radiological appearances in allergic broncho-pulmonary aspergillosis. Clin Radiol. 1970; 21:366–375.
70. Mintzer RA, Rogers LF, Kruglik GD, Rosenberg M, Neiman HL, Patterson R. The spectrum of radiologic findings in allergic bronchopulmonary aspergillosis. Radiology. 1978; 127:301–307.
71. Shah A. Allergic bronchopulmonary aspergillosis. Allergy Clin Immunol Int. 2005; 17:172–180.
72. Shah A. Allergic bronchopulmonary and sinus aspergillosis: the roentgenologic spectrum. Front Biosci. 2003; 8:e138–e146.
73. Shah A, Agarwal AK, Chugh IM. Hilar adenopathy in allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 1999; 82:504–506.
74. Shah A, Bhagat R, Panchal N, Jaggi OP, Khan ZU. Allergic bronchopulmonary aspergillosis with middle lobe syndrome and allergic Aspergillus sinusitis. Eur Respir J. 1993; 6:917–918.
75. Shah A, Behera S, Panjabi C. Middle lobe syndrome: a rare presentation of allergic bronchopulmonary aspergillosis. Eur Ann Allergy Clin Immunol. 2014; 46:147–151.
76. Greenberger PA, Patterson R. Allergic bronchopulmonary aspergillosis. Model of bronchopulmonary disease with defined serologic, radiologic, pathologic and clinical findings from asthma to fatal destructive lung disease. Chest. 1987; 91:165S–171S.
77. Panchal N, Pant C, Bhagat R, Shah A. Central bronchiectasis in allergic bronchopulmonary aspergillosis: comparative evaluation of computed tomography of the thorax with bronchography. Eur Respir J. 1994; 7:1290–1293.
78. Shah A, Pant CS, Bhagat R, Panchal N. CT in childhood allergic bronchopulmonary aspergillosis. Pediatr Radiol. 1992; 22:227–228.
79. Agarwal AK, Bhagat R, Panchal N, Shah A. Allergic bronchopulmonary aspergillosis with aspergilloma mimicking fibrocavitary pulmonary tuberculosis. Asian Pac J Allergy Immunol. 1996; 14:5–8.
80. Goyal R, White CS, Templeton PA, Britt EJ, Rubin LJ. High attenuation mucous plugs in allergic bronchopulmonary aspergillosis: CT appearance. J Comput Assist Tomogr. 1992; 16:649–650.
81. Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A, Jindal SK. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest. 2007; 132:1183–1190.
82. Murphy D, Lane DJ. Pleural effusion in allergic bronchopulmonary aspergillosis: two case reports. Br J Dis Chest. 1981; 75:91–95.
83. Bhagat R, Shah A, Jaggi OP, Khan ZU. Concomitant allergic bronchopulmonary aspergillosis and allergic Aspergillus sinusitis with an operated aspergilloma. J Allergy Clin Immunol. 1993; 91:1094–1096.
84. Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012; 129:280–291.
85. Hutcheson PS, Knutsen AP, Rejent AJ, Slavin RG. A 12-year longitudinal study of Aspergillus sensitivity in patients with cystic fibrosis. Chest. 1996; 110:363–366.
86. Nikolaizik WH, Crameri R, Blaser K, Schöni MH. Skin test reactivity to recombinant Aspergillus fumigatus allergen I/a in patients with cystic fibrosis. Int Arch Allergy Immunol. 1996; 111:403–408.
87. Nikolaizik WH, Weichel M, Blaser K, Crameri R. Intracutaneous tests with recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis and Aspergillus allergy. Am J Respir Crit Care Med. 2002; 165:916–921.
88. Brummund W, Resnick A, Fink JN, Kurup VP. Aspergillus fumigatus-specific antibodies in allergic bronchopulmonary aspergillosis and aspergilloma: evidence for a polyclonal antibody response. J Clin Microbiol. 1987; 25:5–9.
89. Crameri R, Hemmann S, Ismail C, Menz G, Blaser K. Disease-specific recombinant allergens for the diagnosis of allergic bronchopulmonary aspergillosis. Int Immunol. 1998; 10:1211–1216.
90. Knutsen AP, Hutcheson PS, Slavin RG, Kurup VP. IgE antibody to Aspergillus fumigatus recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. 2004; 59:198–203.
91. Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003; 37:Suppl 3. S265–S280.
92. Nichols D, Dopico GA, Braun S, Imbeau S, Peters ME, Rankin J. Acute and chronic pulmonary function changes in allergic bronchopulmonary aspergillosis. Am J Med. 1979; 67:631–637.
93. Panjabi C, Shah A. Lung functions in allergic bronchopulmonary aspergillosis. Respirology. 2006; 11:A38.
94. Patterson R, Greenberger PA, Radin RC, Roberts M. Allergic bronchopulmonary aspergillosis: staging as an aid to management. Ann Intern Med. 1982; 96:286–291.
95. Greenberger PA, Patterson R. Diagnosis and management of allergic bronchopulmonary aspergillosis. Ann Allergy. 1986; 56:444–448.
96. Shah A, Bhagat R, Pant K, Jaggi OP, Khan ZU. Allergic bronchopulmonary aspergillosis with aspergilloma: exacerbation after prolonged remission. Indian J Tuberc. 1993; 40:39–41.
97. Ramachandran S, Shah A, Pant K, Bhagat R, Jaggi OP. Allergic bronchopulmonary aspergillosis and Candida albicans colonization of the respiratory tract in corticosteroid-dependent asthma. Asian Pac J Allergy Immunol. 1990; 8:123–126.
98. Fink JN. Therapy of allergic bronchopulmonary aspergillosis. Indian J Chest Dis Allied Sci. 2000; 42:221–224.
99. Rosenberg M, Patterson R, Roberts M, Wang J. The assessment of immunologic and clinical changes occurring during corticosteroid therapy for allergic bronchopulmonary aspergillosis. Am J Med. 1978; 64:599–606.
100. Shah A, Panjabi C. Biweekly therapy with prednisolone is effective in the management of allergic bronchopulmonary aspergillosis [abstract]. Allergy Clin Immunol Int. 2005; Suppl 1. 113.
101. Thomson JM, Wesley A, Byrnes CA, Nixon GM. Pulse intravenous methylprednisolone for resistant allergic bronchopulmonary aspergillosis in cystic fibrosis. Pediatr Pulmonol. 2006; 41:164–170.
102. Leon EE, Craig TJ. Antifungals in the treatment of allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 1999; 82:511–516.
103. Wark PA, Gibson PG, Wilson AJ. Azoles for allergic bronchopulmonary aspergillosis associated with asthma. Cochrane Database Syst Rev. 2004; 3:CD001108.
104. Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, et al. Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009; 15:1068–1076.
105. Chishimba L, Niven RM, Cooley J, Denning DW. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012; 49:423–433.
106. Clancy CJ, Nguyen MH. Long-term voriconazole and skin cancer: is there cause for concern? Curr Infect Dis Rep. 2011; 13:536–543.
107. van der Ent CK, Hoekstra H, Rijkers GT. Successful treatment of allergic bronchopulmonary aspergillosis with recombinant anti-IgE antibody. Thorax. 2007; 62:276–277.
108. Kanu A, Patel K. Treatment of allergic bronchopulmonary aspergillosis (ABPA) in CF with anti-IgE antibody (omalizumab). Pediatr Pulmonol. 2008; 43:1249–1251.
109. Tillie-Leblond I, Germaud P, Leroyer C, Tétu L, Girard F, Devouassoux G, et al. Allergic bronchopulmonary aspergillosis and omalizumab. Allergy. 2011; 66:1254–1256.
110. Collins J, Devos G, Hudes G, Rosenstreich D. Allergic bronchopulmonary aspergillosis treated successfully for one year with omalizumab. J Asthma Allergy. 2012; 5:65–70.
111. Agarwal R, Hazarika B, Gupta D, Aggarwal AN, Chakrabarti A, Jindal SK. Aspergillus hypersensitivity in patients with chronic obstructive pulmonary disease: COPD as a risk factor for ABPA? Med Mycol. 2010; 48:988–994.
112. Mir E, Shah A. Allergic bronchopulmonary aspergillosis in a patient with chronic obstructive pulmonary disease. Prim Care Respir J. 2012; 21:111–114.
113. Shah A, Panchal N, Agarwal AK. Concomitant allergic bronchopulmonary aspergillosis and allergic Aspergillus sinusitis: a review of an uncommon association. Clin Exp Allergy. 2001; 31:1896–1905.
114. Sharma P, Agarwal AK, Shah A. Formation of an aspergilloma in a patient with allergic bronchopulmonary aspergillosis on corticosteroid therapy. Indian J Chest Dis Allied Sci. 1998; 40:269–273.
115. Shah A, Panjabi C. Contemporaneous occurrence of allergic bronchopulmonary aspergillosis, allergic Aspergillus sinusitis, and aspergilloma. Ann Allergy Asthma Immunol. 2006; 96:874–878.
116. Katzenstein AL, Sale SR, Greenberger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol. 1983; 72:89–93.
117. Shah A, Khan ZU, Sircar M, Chaturvedi S, Malik GB, Randhawa HS. Allergic Aspergillus sinusitis: an Indian report. Respir Med. 1990; 84:249–251.
118. Shah A, Sircar M. Sensitization to Aspergillus antigens in perennial rhinitis. Asian Pac J Allergy Immunol. 1991; 9:137–139.
119. Sahay S, Shah A. Allergic rhinitis: aspergillus sensitisation increases the severity of sinusitis in "blockers" as compared to "sneezers and runners" [abstract]. Allergy. 2008; 63:73.
120. Panjabi C, Shah A. Allergic Aspergillus sinusitis and its association with allergic bronchopulmonary aspergillosis. Asia Pac Allergy. 2011; 1:130–137.
121. Ein ME, Wallace RJ Jr, Williams TW Jr. Allergic bronchopulmonary aspergillosis-like syndrome consequent to aspergilloma. Am Rev Respir Dis. 1979; 119:811–820.
122. Shah A. Concurrent allergic bronchopulmonary aspergillosis and aspergilloma: is it a more severe form of the disease? Eur Respir Rev. 2010; 19:261–263.
123. Godet C, Philippe B, Laurent F, Cadranel J. Chronic pulmonary aspergillosis: an update on diagnosis and treatment. Respiration. 2014; 88:162–174.
124. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015; 70:270–277.
125. Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011; 37:865–872.
126. Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res. 2015; 7:205–220.
127. Shah A. Asthma and aspergillus. Indian J Chest Dis Allied Sci. 2004; 46:167–170.
128. Diwakar A, Panjabi C, Shah A. Allergic bronchopulmonary aspergillosis, allergic Aspergillus sinusitis and their co-occurrence. Open Allergy J. 2008; 1:52–61.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download