Abstract
Purpose
Subcutaneous allergen-specific immunotherapy (SCIT) is a well-established and clinically effective method to treat allergic diseases, such as rhinitis and asthma. It remains unclear how soon after initiation of an ultra-short course of grass pollen immunotherapy adjuvanted with monophosphoryl lipid A (MPL)-specific bronchial tolerance can be induced.
Methods
In a prospective study of 69 children double-sensitized to birch and grass pollens (51 males, average age 11.1 years), development of bronchial tolerance after 1 cycle of SCIT for grass was evaluated. In all the patients, the bronchial allergen provocation test (BAP) was performed before and after treatment. According to the results of the first BAP, the patients were divided into 2 groups: those showing a negative BAP with a decrease in FEV1 of <20% (seasonal allergic rhinitis [SAR] group, n=47); and those showing a positive BAP with a decrease in FEV1 of ≥20% (SAR with allergic asthma [SAR and Asthma] group, n=22). All the patients received MPL-adjuvanted, ultra-short course immunotherapy for birch, but only those with a positive BAP to grass received MPL-SCIT for grass.
Results
After the pollen season, the BAP in the SAR group remained unchanged, while it was improved in the SAR and Asthma group (decrease in FEV1 of 28.8% vs 12.5%, P<0.01). The IgG4 levels increased after SCIT (median before SCIT 0.34 to 11.4 after SCIT), whereas the total and specific IgE levels remained unchanged.
Atopic diseases, such as allergic rhinitis and asthma, affect up to 30% of the population in Western countries.1 Allergen-specific immunotherapy (SIT) is the only treatment available that has the potential to ameliorate symptoms and to reduce or abrogate allergic diseases.23 The success rates vary substantially depending on the allergen, the route of administration, and the dosing regimen.456 To date, SIT has encompassed different dosing regimens and administration routes. Conventional SIT involves up-dosing, followed by monthly injections over 3 years, resulting in up to 50 injections. To make immunotherapy more acceptable, short and ultra-short course pre-seasonal regimens have been developed. The development of allergoids has allowed for a higher dose of allergen with every injection. Pre-seasonal regimens containing allergoids have previously been shown to be effective for pollen allergies.78 These new regimens have not yet been tested in prospective, randomized head-to-head studies with conventional preparations. Moreover, little is known about the required doses and duration of therapy to achieve a rapid and sustained treatment effect. The design of objective studies is difficult because changes in laboratory markers, such as IgE and IgG4, and deviations in T-cell proliferation91011 are not directly correlated with clinical changes. Therefore, efficacy has mainly been assessed using symptom and medication scores, quality of life questionnaires, and evaluations by physicians before and after treatment.9 Unfortunately, all of these scores have failed to reliably demonstrate significant improvement after 1 year of treatment, due to the changing pollen counts from season to season.2 Allergen-specific provocation tests, such as conjunctival, nasal, and bronchial challenges, are now being used more frequently to measure treatment success objectively. Importantly, these tests are only minimally influenced by placebo effects. In a Cochrane review by Abramson,12 bronchial allergen provocation (BAP) was particularly recommended for determining the success of SIT. Using BAP, changes in bronchial sensitivity to a certain allergen can be accurately assessed.1314 This method was assessed in adults and in children, and we were able to demonstrate the safety and feasibility even in young children.
One of the pre-seasonal allergen preparations is an ultra-short course allergy vaccine that requires only 4 injections per year. It contains glutaraldehyde-modified allergoids linked to L-tyrosine and to the TH1-inducing adjuvant monophosphoryl-lipid-A (MPL).14 Clinical experience with pollen allergoids adjuvanted with MPL has been extensive, with more than 5,000 subjects treated in clinical and post-marketing studies and approximately 220,000 patients receiving the drug until now.101516 However, little is known about the drug's onset of action after 1 course of treatment, which is administered before the start of the pollen season.
In this study, we compared treatment success after 1 course of MPL-SCIT against grass by BAP before and after treatment to that of an untreated control group.
Sixty-nine patients (51 males, 18 females, range 5 to 17 years, mean age 11.1 years) were enrolled in the study (Table). The patients were selected from the Outpatient Department of Pediatric Allergy, Pneumology, and Cystic Fibrosis in the Children's Hospital of Goethe-University in Frankfurt/Main, Germany. All patients had a history of at least 1 year of allergic symptoms during the birch- and grass-pollen season and no previous immunotherapy. The patients predominantly had allergic rhinitis or rhinoconjunctivitis and coughing during the pollen season. In none of the patients, moderate or persistent asthma was previously diagnosed. They showed only minimal asthma symptoms (cough, wheeze) that were reflected by the absence of controller therapy in this cohort. Inclusion criteria were as follows: signed informed consent; participants at >5 and ≤18 years of age who had a clinical history of seasonal allergic rhinoconjunctivitis (SAR) and/or allergic asthma grade I, according to the GINA guidelines, during the birch and grass pollen seasons; elevated serum allergen-specific IgE (RAST ≥ II or ≥0.7 kU/L) to birch and grass pollens, and no significantly abnormal findings on physical examination. In addition, a positive response to the bronchial allergen challenge with grass pollen (FEV1 fall of ≥20%) was arbitrarily defined as allergic asthma.
Reasons for exclusion included the following: FEV1 less than 70% of the predicted value prior to enrollment, concomitant therapy with inhaled corticosteroids (ICS) for at least 3 months in duration, the use of systemic steroids, and the regular use of antihistamines. Additional patients with serious underlying conditions were also excluded from the study.
All the parents and all patients at ≥14 years of age provided written informed consent.
This study is a non-randomized open trial that encompassed 2 study visits. At visit 1, a complete medical history, including concomitant diseases and medications, a physical examination, measurement of exhaled nitric oxide (FeNO), and spirometry were performed. In patients meeting the inclusion criteria, blood samples were obtained (see parameters below). All the patients included in this study underwent a single-step BAP with 30 AU of grass pollen. A decrease in FEV1 of ≥20% was defined as "positive" as described before.13 The patients were assigned to the SAR group (decrease in FEV1 <20%) or the SAR and Asthma group (decrease in FEV1 ≥20%) according to the results of BAP. Additionally, the patients received a symptom diary to be completed over 2 weeks during the grass pollen season (see questionnaire below). In the SAR group, visit 2 was performed after the grass pollen season approximately 1 year after the first BAP. In the SAR and Asthma group, visit 2 was performed 4 to 6 weeks after the end of MPL-SCIT against grass pollen. The second BAP was done in different time intervals in both groups due to ethical reasons. Our ethics committee did not give permission to repeat a negative BAP earlier.
Research ethics approval was granted by the local ethics committee of Goethe University in Frankfurt/M, Germany (approval No. 237/07).
All the patients received specific subcutaneous immunotherapy (SCIT) with a preparation containing an L-tyrosine-adsorbed birch allergoid with MPL (Pollinex® quattro, Bencard, Munich, Germany, subsidiary of Allergy therapeutics, Worthing, West Sussex, UK).15 At weekly intervals, the patients received 300, 800, 2,000, and 2,000 units subcutaneously. The SAR and Asthma group was additionally treated with a preparation containing an L-tyrosine-adsorbed Phleum pratense allergoid (Pollinex® quattro, Bencard, Munich, Germany).
Baseline spirometry (before inhalation of sterile saline 0.9% and before BAP) was performed using a MasterScreen® body plethysmograph manufactured by VIASYS Healthcare GmbH (Hoechberg, Germany). Before and 0-8 hours after BAP, the subjects measured FEV1 hourly using a SpiroPro® manufactured by VIASYS Healthcare GmbH. These data were not evaluated, but were only obtained for safety reasons. In case of a decrease in FEV1 of greater than 20%, the subjects were asked to use a short-acting beta-2-agonist (SABA).
Solutions of grass allergen extract (Allergopharma, Reinbek, Germany) were prepared according to the instruction manual. The lyophilized grass allergen extract was resuspended in the solution provided by the manufacturer (containing sodium, phenol, and water), resulting in aliquots of 5,000 mg/mL; the aliquots were stored immediately after preparation at 4℃ until applied. All the subjects were asked to inhale sterile saline 0.9%, followed 5 minutes later by a single-step inhalation of 30 AU of grass pollen. The solutions were delivered via a medical aid nebulizer and an aerosol provocation system (APS®) powered by compressed air (VIASYS Healthcare GmbH). The post-saline FEV1 was set as the baseline value, and a decrease in FEV1 after a single-step BAP (30 AU of grass pollen allergen) was expressed as a percentage change from baseline.
To ensure safety, all the patients received a peak flowmeter for peak expiratory flow (PEF) measurement at home. PEF was measured for up to 10 hours following the challenge, and a late airway response was defined as a PEF decrease of ≥15%. The subjects received an action plan and rescue medication consisting of salbutamol and prednisolone 50 mg. This procedure was developed for earlier studies1316 and was found to be safe and able to detect potential side effects as early as possible.
The measurements were made using the NIOX® (Aerocrine, Solna, Sweden), which measures FeNO in exhaled air according to the ATS guidelines.17
Blood samples were obtained each study visit. The total and specific IgE, responses to grass and birch were analyzed using a 2-sided chemiluminescent assay (Immulite 2,000, Siemens AG Healthcare, Erlangen, Germany). Specific IgG4 levels were determined using ELISA (Diagnostic Systems & Technologies GmbH, Schwerin, Germany). The analysis was performed according to the manufacturer's instructions.
During the grass pollen season, the patients were asked to record their symptoms and medication use in their symptom diaries (recorded from the 16th until the 22nd of May and from the 13th until the 19th of June―at the beginning and during the peak of the grass pollen season). The documentation encompassed 5 nasal and conjunctival symptoms (runny nose, blocked nose, itchy nose, sneezing, and itchy eyes) and 4 asthma symptoms (shortness of breath, wheezing, chest tightness, and coughing). Each item was rated from "no symptoms" (0) to "severe symptoms" (3). Furthermore, the use of the following medications was recorded and weighted as follows: disodium chromoglycate (1), antihistamines (1), beta agonists (1), leukotriene antagonists (4), topical steroids (3), and systemic steroids (6). The scores were totaled for the weekly overall score (WOS) with a range of 0-139. The WOSs of both weeks were summed. In total, the WOSs of both weeks therefore had a range of 0-278.
The primary endpoint was improvement in a decrease in FEV1 after single-step allergen challenge with 30 AU of grass pollen. The level of statistical significance was set at a P value of less than 0.05.
The secondary endpoints were changes in quality of life and the use of allergen-specific medication. The data were statistically analyzed and graphed with GraphPad Prism software, version 5.01 (GraphPad Software Inc., La Jolla, CA, USA). The maximum decrease in FEV1 after each BAP and the FeNO value after a single-step challenge are expressed as median and range. For the comparison of before/after values, the paired t test (Wilcoxon's matched t test) was used. For the analysis of the current medication and clinical complaints, a 4-field table test (Fisher's exact test) was used. The logarithmic graphs are shown in log10.
Data from a previous study showed that the early asthmatic response (EAR) had a standard deviation of approximately 11% (root mean square error from ANOVA with baseline correction). With 14 evaluable subjects in each treatment group, either of the 2 t tests described above had approximately 90% power to detect a difference in EAR of 14% between active treatment and placebo.18
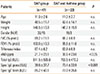
We were able to enroll 69 SAR patients double sensitized to birch and grass. All the patients received MPL-SCIT against birch. The patients were classified into 2 groups: the SAR group-untreated control group (n=47, decrease in FEV1 <20%); and the SAR and Asthma group―treatment group (n=22, decrease in FEV1 ≥20%) according to the BAP results (see below). The patients were well matched for age, height, weight, and total IgE, as summarized in Table. As expected in patients with SAR and asthma, the median FeNO and specific IgE grass values were significantly higher (Table). Thirty-eight patients in the SAR group and 19 patients of the SAR and Asthma group completed the study according to the protocol (Fig. 1).
Baseline lung function (vital capacity, forced expiratory volume in 1 second, Tiffeneau-Index) was within the normal range (>70% pred) and showed no significant differences between both groups at visits 1 or 2 (Table).
In the SAR group receiving only MPL-SCIT against birch, the specific bronchial reactivity to grass remained unchanged between visits 1 and 2. The median decrease in FEV1 after the first BAP (4.3%, range 18.4%-15.1%) remained unchanged compared to the second BAP (5.9%, range, 39.1%-2.1%). Interestingly, 3 patients in the SAR group had a significant decrease of FEV1 >20% at the second BAP, indicating that there was a small subgroup of SAR patients (8.1%) that was deteriorating rapidly and more prone to develop allergic asthma.
In the SAR and Asthma group (n=22), we were able to demonstrate statistically significant improvement 4 to 6 weeks after active treatment. The median decrease in FEV1 after BAP with 30 AU of grass was 28.8% (57.0%-20.2%) before treatment and 12.5% (57.0% to -6.6%) after MPL-SCIT (Fig. 2, P<0.01). The overall response after 1 cycle of MPL-SCIT was 63.2 % (number of patients without a significant decrease in FEV1). However, the results were not homogeneous: of 19 patients with complete data in this treatment group, 14 improved (decrease in FEV1), while 1 remained unchanged and 4 deteriorated.
In the SAR group, the FeNO levels were 21.6 ppb (±23.1 ppb, n=47) at baseline and 37.4 ppb (±46.2 ppb, n=39) 1 year later. In the SAR and Asthma group, the FeNO levels were 30.6 ppb (± 30.6, n=22) at baseline and 29.3 ppB (±22.8 ppb, n=19) after the SCIT. The differences did not reach statistical significance.
The levels of total and specific IgE were similar in both groups and remained unchanged. In the SAR group, the mean total IgE was 611±586 kU/L, and the mean specific IgE for grass were 39.6±37.7 kU/L at baseline and 747±924 kU/L and 43.1±39.0 kU/L, respectively, after 1 year. These results were similar in the SAR and Asthma group: total/specific IgE was 703±472 and the mean specific IgE for grass was 73.4±33.9 kU/L at baseline, and 687±525 and 67.8±36.1 kU/L, respectively, after SCIT. These changes did not attain statistical significance (Fig. 3).
As a surrogate marker for successful SCIT, the IgG4 levels increased from a median of 0.3 kU/L (0.3 to 33.2) to 11.4 kU/L (0.3 to 101.0) after SCIT in the SAR and Asthma group (Fig. 4).
The diary return rates in the SAR group was 87.2% (41/47) in May and 76.6% (36/47) in June, which was comparable to those in the SAR and Asthma group, with 77.3% (17/22) and 81.8% (18/22) in May and June, respectively. The mean WOSs in the SAR group were 34.6±30.3 and 29.5±31.1 in May and June, respectively, which were similar to those in the SAR and Asthma group (34.0±24.0 and 32.3±26.2, respectively).
The utilization of symptomatic medication in May and June was higher in the SAR group, with scores of 7.5±12.3 and 7.3±11.9, respectively, compared to 5.4±9.7) and 4.8±10.1 in the SAR and Asthma group.
We were able to show that MPL-SCIT against grass induced bronchial tolerance in patients with grass pollen allergies after 1 course with only 4 injections. The bronchial hyperactivity measured by BAP with 30 AU of grass pollen was almost halved (mean decrease in FEV1: 28.8% [57.0% to 20.2%] before treatment to 12.5% [57.0% to -6.6%] after MPL-SCIT, P<0.01). Interestingly, in 12 patients BHR to the allergen was completely abolished, while 2 patients improved slightly and 5 deteriorated, emphasizing a great variation in individual response to SCIT. The overall response of 63.2% after the first year was an excellent and quite surprising result. Many conventional SCIT extracts and dosage regimens have failed to show significant improvement after the first year of treatment.1920 The rapid onset of 1 course of MPL-SCIT against grass is fascinating and in keeping with a recent study of allergic rhinitis.21 The authors of that study evaluated the clinical efficacy and safety of ragweed MPL compared to a placebo using controlled ragweed pollen exposure. After only 1 course of MPL-SCIT against ragweed, the mean improvement in total symptom scores was significantly greater in the ragweed group than in the placebo group.
The present study was designed solely to evaluate the effects of grass MPL on allergic asthmatic patients using BAP. Recent technical advances have facilitated the measurement of BAP, making it reliable, reproducible, easy to perform, and safe.1316 The non-randomized design, i.e., with all patients with BAP of >20% receiving treatment, and the small sample size studied were not expected to result in significant differences between the symptom scores of treated and untreated patients. Moreover, it is well known that symptom scores are profoundly influenced by placebo effects.22 In addition, all the patients received SCIT with MPL birch, which might explain why the symptom scores were almost identical in the 2 groups. This study supported the use of allergen provocation models to objectify treatment success in SIT because these models, in contrast to symptom and medication scores, have objectively demonstrated treatment success in small cohorts by comparing individual reactions before and after SIT at the affected organs.121423 Furthermore, this study compared established markers of treatment success, including specific IgE and IgG4, to the results of standardized BAP in children. As expected, IgG4 significantly increased, whereas IgE was not changed. There is an ongoing discussion between clinicians regarding treatment regimens that both are feasible for patients and can best induce tolerance. The major disadvantages of conventional SCIT are the frequency of injections and the risk of systemic adverse events. Nevertheless, many clinicians favor conventional over short-term or pre-seasonal SCIT because of its traditional use and the availability of long-term data.242526 In addition, there is a strong belief that the total amount of allergen delivered is correlated with sustained treatment success. However, only a few trials have shown clear evidence that a greater amount of allergen for pre-seasonal vs conventional treatment was favorable.2728 In contrast, 2 recent studies, comparing 3-year treatment to 5-year treatment, showed no benefits with longer treatment, challenging the concept that longer therapy is superior to short-term treatment.2930
Due to rapid advances in the field of immunotherapy, there has been an increasing need to objectively assess treatment successes with different allergen extracts and treatment regimens. In particular, MPL represents a promising adjuvant for SCIT, by inducing a strong TH1 response with relatively little associated toxicity. Numerous clinical trials and treatment reports have found that MPL-containing extracts was well-tolerated and resulted in significant reductions in symptom scores, poor provocation test results, and antigen-specific IgE value, as well as increased antigen-specific IgG/IgG4, after only 1 or 2 courses.11
The rapid onset of action was an important finding for affected patients because there are only a few studies available so far that have addressed the onset of protection against asthma symptoms after SIT.31 In the PAT study, the onset was measured after 3 years. Even stronger protection against asthma, with a faster onset of action, has been described in recent SLIT studies.3132 In addition to the protection it affords against allergic symptoms, SIT is believed to facilitate long-lasting carry-over effects after the discontinuation of treatment.333435 However, 1 cycle of short-course immunotherapy might not be able to induce long-term effects, because bronchial tolerance, the induction of T reg cells, and specific IgG4 values require repeated training (allergy review) and also because a weakness of our study was that the results were not confirmed in follow-ups at the second and third years.
To our knowledge, this is the first study in which patients with allergies to birch and grass were either treated against both allergies or received SIT against birch only. Recently, the question was raised whether SIT has unspecific protective effects. Epidemiological studies and experimental models have suggested an unspecific protection against allergies by strong Th-1 activators such as MPL.3637 However, we were not able to demonstrate any cross-reactivity in the untreated controls; in fact, bronchial tolerance to grass pollen decreased significantly in the group only being treated with a birch preparation.
Figures and Tables
Fig. 1
Randomization and follow-up. Of the 69 patients included, 57 completed the study. The dropout occurred because the patients or their legal representatives refused to undergo a second BAP or were lost to follow-up.
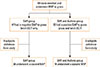
Fig. 2
Decreases in FEV1 after the first and second BAPs. Decreases in FEV1 after BAP with grass pollen at baseline (first and third columns) and after the second BAP (second and fourth columns) in the SAR group and the MPL-SCIT treated SAR and Asthma group, respectively.

Fig. 3
Total IgE and specific IgE against grass pollen. Total IgE and specific IgE against grass pollen at baseline (first and third columns) and in the SAR group after 1 year and the MPL-SCIT treated SAR and Asthma group (second and fourth columns, respectively).
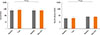
Fig. 4
Specific IgG against grass pollen. Grass-specific IgG4 before and after SCIT in the MPL-SCIT treated SAR and Asthma group.
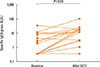
Table 1
Patient characteristics
ACKNOWLEDGMENTS
Stefan Zielen received lecture fees from several companies, including Bencard, Allergopharma, ALK, Novartis, GSK, Böhringer, Vifor, Bionorica and Biotest.
The study was partially sponsored by Bencard of Munich, Germany.
References
1. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006; 355:2226–2235.
2. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3.
3. Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015; 7:221–229.
4. Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of hymenoptera venom hypersensitivity: a meta-analysis. Clin Ther. 2000; 22:351–358.
5. Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001; 31:1392–1397.
6. Kim ST. Outcome of sublingual immunotherapy in patients with allergic rhinitis sensitive to house dust mites. Allergy Asthma Immunol Res. 2015; 7:99–100.
7. Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001; 56:498–505.
8. Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Study Group. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005; 60:801–807.
9. Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007; 62:317–324.
10. Shamji MH, James LK, Durham SR. Serum immunologic markers for monitoring allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2011; 31:311–323. x
11. Rosewich M, Schulze J, Eickmeier O, Telles T, Rose MA, Schubert R, et al. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 2010; 160:403–410.
12. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010; CD001186.
13. Schulze J, Rosewich M, Dressler M, Riemer C, Rose MA, Zielen S. Bronchial allergen challenge using the medicaid dosimeter. Int Arch Allergy Immunol. 2012; 157:89–97.
14. Rosewich M, Arendt S, El Moussaoui S, Schulze J, Schubert R, Zielen S. Bronchial allergen provocation: a useful method to assess the efficacy of specific immunotherapy in children. Pediatr Allergy Immunol. 2013; 24:434–440.
15. Rosewich M, Lee D, Zielen S. Pollinex Quattro: an innovative four injections immunotherapy in allergic rhinitis. Hum Vaccin Immunother. 2013; 9:1523–1531.
16. Rosewich M, Rose MA, Eickmeier O, Travaci M, Kitz R, Zielen S. Montelukast as add-on therapy to beta-agonists and late airway response. Eur Respir J. 2007; 30:56–61.
17. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.
18. Schulze J, Reinmüller W, Herrmann E, Rosewich M, Rose MA, Zielen S. Bronchial allergen challenges in children - safety and predictors. Pediatr Allergy Immunol. 2013; 24:19–27.
19. Kleine-Tebbe J, Walmar M, Bitsch-Jensen K, Decot E, Pfaar O, de Rojas DH, et al. Negative clinical results from a randomised, double-blind, placebo-controlled trial evaluating the efficacy of two doses of immunologically enhanced, grass subcutaneous immunotherapy despite dose-dependent immunological response. Clin Drug Investig. 2014; 34:577–586.
20. Kuehr J, Brauburger J, Zielen S, Schauer U, Kamin W, Von Berg A, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002; 109:274–280.
21. Patel P, Holdich T, Fischer von, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014; 133:121–129.e1-2.
22. Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, Kirsch I, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011; 365:119–126.
23. Horak F, Toth J, Hirschwehr R, Marks B, Stübner UP, Jäger S, et al. Effect of continuous allergen challenge on clinical symptoms and mediator release in dust-mite-allergic patients. Allergy. 1998; 53:68–72.
24. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007; 62:943–948.
25. Eng PA, Reinhold M, Gnehm HP. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy. 2002; 57:306–312.
26. Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999; 341:468–475.
27. Frew AJ, Powell RJ, Corrigan CJ, Durham SR. UK Immunotherapy Study Group. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006; 117:319–325.
28. Tworek D, Bochenska-Marciniak M, Kuprys-Lipinska I, Kupczyk M, Kuna P. Perennial is more effective than preseasonal subcutaneous immunotherapy in the treatment of seasonal allergic rhinoconjunctivitis. Am J Rhinol Allergy. 2013; 27:304–308.
29. Stelmach I, Sobocińska A, Majak P, Smejda K, Jerzyńska J, Stelmach W. Comparison of the long-term efficacy of 3- and 5-year house dust mite allergen immunotherapy. Ann Allergy Asthma Immunol. 2012; 109:274–278.
30. Tabar AI, Arroabarren E, Echechipía S, García BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. The J Allergy Clin Immunol. 2011; 127:57–63. 63.e1–63.e3.
31. Marogna M, Tomassetti D, Bernasconi A, Colombo F, Massolo A, Businco AD, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008; 101:206–211.
32. Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004; 114:851–857.
33. Jacobsen L, Wahn U, Bilo MB. Allergen-specific immunotherapy provides immediate, long-term and preventive clinical effects in children and adults: the effects of immunotherapy can be categorised by level of benefit -the centenary of allergen specific subcutaneous immunotherapy. Clin Transl Allergy. 2012; 2:8.
34. Madonini E, Agostinis F, Barra R, Berra A, Donadio D, Pappacoda A, et al. Long-term and preventive effects of sublingual allergen-specific immunotherapy: a retrospective, multicentric study. Int J Immunopathol Pharmacol. 2003; 16:73–79.
35. Compalati E, Braido F, Canonica GW. An update on allergen immunotherapy and asthma. Curr Opin Pulm Med. 2014; 20:109–117.
36. Gerhold K, Bluemchen K, Franke A, Stock P, Hamelmann E. Exposure to endotoxin and allergen in early life and its effect on allergen sensitization in mice. J Allergy Clin Immunol. 2003; 112:389–396.
37. Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014; 133:551–559.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download