Abstract
Purpose
Questionnaire-based diagnostic criteria for atopic dermatitis (AD) have been proposed to detect the major group of AD with flexural dermatitis. We aimed to develop novel, questionnaire-based diagnostic criteria for childhood AD, which can detect more comprehensive AD including non-flexural type.
Methods
The draft version of questionnaire to detect childhood AD was prepared to be used for preliminary hospital- (n=1,756) and community-based (n=1,320) surveys. From analysis, the Reliable Estimation of Atopic dermatitis of ChildHood (REACH) was derived and verified in derivation (n=1,129) and validation (n=1,191) sets by community-based surveys.
Results
The REACH consists of 11 questions including 2 major and 9 minor criteria. AD is diagnosed as the major group of 'eczema on the antecubital or popliteal fossa' to fulfill the 2 major criteria (2M), and the minor group of 'eczema on the non-antecubital or popliteal fossa' to fulfill the 1 major plus 4 or more minor criteria (1M+4m). In the validation set, the overall 1-year AD prevalence by the REACH was estimated as 12.3% (95% CI, 10.5%-14.2%), and the REACH showed a sensitivity of 75.2%, a specificity of 96.1%, and an error rate of 6.4%. The REACH demonstrated better diagnostic performance than the ISAAC in terms of the number of misclassification (10.0%).
Various diagnostic tools for atopic dermatitis (AD) have been proposed due to the lack of definitive biomarkers and the marked diversity of its clinical features.12 Hanifin and Rajka's criteria were developed for AD diagnosis at the individual level in a hospital setting.3 As the criteria were based on the empirical experiences of AD experts, they encompass numerous, minor features unfamiliar to untrained examiners. Subsequently, the UK Working Group refined the criteria to improve their practical applicability (UK's criteria) that included a minimum set of valid and reliable items for use in both hospital and community settings.456 Meanwhile, questionnaire-based diagnostic criteria for AD, including the International Study for Asthma and Allergies in Childhood (ISAAC), were developed for epidemiological surveys in a community setting.1789
Hospitals-detected AD patients who are diagnosed in the hospitals are known to have more severe symptoms than those who are detected by community-based surveys. In previous studies on the severity of AD, 67.9%-86.1% of AD patients were mild in community-based surveys, while 32.7% of AD patients were mild in hospital-based surveys.10111213 Interestingly, 36% of AD schoolchildren, who were detected by community-based surveys, did not consulted doctors (probably with mild AD), suggesting the importance of epidemiological surveys in given communities.11 In fact, the majority of participants (i.e., both children and their parents) have a strong desire to be consulted on their skin conditions at the individual level through epidemiological surveys. Taken together, it is suggested that continuous monitoring on AD prevalence in given communities or individuals are very important for efficient AD management.
As a simple, convenient diagnostic tool for epidemiological surveys, the ISAAC was designed mainly to detect the major group of AD with flexural dermatitis. Therefore, it has a limit to detect the minor group of AD, such as non-flexural, nummular-type AD.21415 Furthermore, the ISAAC is not supposed to differentiate non-AD diseases from itchy skin eruptions, such as urticaria due to its simplified questions on flexural dermatitis. The Korean Atopic Dermatitis Association (KADA) aimed to devise more comprehensive, accurate, questionnaire-based diagnostic criteria for AD, for use not only in the context of epidemiological surveys, but also to provide medical advice at individual levels.
For this purpose, we designed new diagnostic criteria for childhood AD; i.e., Reliable Estimation of Atopic dermatitis in ChildHood (REACH), encompassing questions not only on relapsing dermatitis (flexural or non-flexural), but also on AD-related factors (atopy history and environmental or aggravating factors). The REACH was developed using a derivation set including both elementary school (7-12 years of age) and preschool (4-6 years of age) children. The criteria were then further validated using a different validation set covering the same age group (Figure). We propose new full-questionnaire-based REACH criteria for childhood AD, which can be used as a different instrument for epidemiological surveys of childhood AD in future.
As a gold standard for the 1-year AD prevalence, dermatologists' skin examination should be performed at least twice a year for the same group of children. For this study, we distributed the draft or final version of questionnaires together with the ISAAC prior to skin examination and performed the first skin examination by 2 dermatologists in June-July. All questionnaires of the draft and final version were answered by parents. From the skin examination, children were classified into 1 of the 3 groups of 'AD', 'non-AD', and 'undetermined'. In November-December, the second skin examination was performed on children, who were classified as 'undetermined' on the first skin examination. Children, who were classified as 'non-AD' by the first skin examination, but were positive to the ISAAC, were also enrolled for the second skin examination. The recruited dermatologists were well informed about the Hanifin and Rajka's criteria3 and KDA's criteria.16
The major groups of AD, which manifested with eczematous skin lesions on the antecubital or popliteal fossa, were classified as 'eczema on the antecubital/popliteal fossae.' The minor group of AD, which manifested with eczematous skin lesions on other locations except the antecubital or popliteal fossa, were classified as 'eczema on the non-antecubital/popliteal fossae.
Twelve dermatologists, who were appointed as members of task force team (TFT), extracted candidate questions on our draft version from the Hanifin and Rajka's,3 ISAAC's,7 and KDA's16 criteria. To prepare questionnaire-based diagnostic criteria for AD, we excluded items requiring invasive laboratory tests, such as 'elevated serum IgE' and 'immediate skin test reactivity.' We also excluded items that were regarded as incomprehensible for people without medical background; pityriasis alba, white dermatographism, etc. The selected items excepted them were classified into major and minor categories following our panel discussion, and based on experts' opinions. In total, 18 questions were classified into 3 sets: I) 5 questions pertaining to recurrent skin rashes on the flexural locations (candidates for major criteria; Q1-1 to Q1-5) and 3 questions were on atopic history (candidates for major criteria; Q1-6); II) 5 questions were on non-flexural dermatitis (candidates for minor criteria; Q2-1 to O2-5); and III) 5 questions on environmental or aggravating factors (candidates for minor criteria; Q3-1 to Q3-5). Among candidate items for major criteria, questions on flexural dermatitis were selected from ISAAC's criteria, and those on atopy history were selected from Hanifin and Rajka's and KDA's criteria.3716 Atopy history was regarded as positive, when subjects responded 'Yes' to at least 1 of the 3 questions on AD, asthma, or allergic rhinitis. All questions were refined using non-medical language that was comprehensible to the general public (Table 1).
For preparing the final version of questionnaire, we performed our surveys in both hospital- and community-settings to select items from candidate questions of the draft version. For the hospital-based survey, ethics approval was obtained from 53 tertiary referral hospitals in Korea. A total of 1,342 children with AD (mean age: 7.8 years), and 414 control children with non-AD skin disorders (mean age: 7.4 years) were enrolled. Non-AD skin disorders included urticaria (62.6%), contact dermatitis (30.5%), and others (6.9%).
For the community-based surveys, 5 elementary schools located in Gwangju City, South Korea (125:54E, 35:09N) were enrolled. All elementary schoolchildren (7-12 years of age) who had submitted their completed parental consent forms before skin examination were enrolled. Among 1,545 elementary schoolchildren, 1,320 (mean age: 10.2 years) were finally enrolled in the study, which corresponds to an 85.4% response rate. From skin examination (twice) in together with the ISAAC, we allocated 149 participants (11.3%) to the AD group; the remaining 1,171 were allocated to the non-AD control group.
The questionnaire established herein (REACH) were administered to 1,129 children in 2 elementary schools and 3 preschools, which were used as a derivation set. To simplify the questionnaire of the REACH, 3 questions on 'personal or family history of atopy (AD, asthma, and allergic rhinitis)' in the draft version were changed into 1 question by putting them together. All combination sets of 'eczema on the antecubital/popliteal fossae' and 'eczema on the non-antecubital/popliteal fossae' according to the respective minimum requirements of the number of minor criteria were evaluated for diagnostic accuracy. Next, we derived new diagnostic criteria considering the accuracy of the estimated AD prevalence, low error rate (ER), and high Youden's index (sensitivity+specificity - 1).
The REACH and ISAAC criteria were applied to 1,191 children drawn from 2 elementary schools and 3 preschools, which were used as a validation set. We compared the estimated AD prevalence and diagnostic accuracy of the REACH with those of the ISAAC. For this report, questions in the REACH were translated into English by 2 bilingual dermatologists, and back-translated into Korean by 2 other bilingual dermatologists to ensure consistency.
From the analysis on the results of the draft version of questionnaire, TFT decided the cutoff value for major criteria as 70% of Youden's index, as the value of 2 questions was markedly different with other questions. 'Recurrent attacks of itchy rash during the last year' represented the most sensitive question (sensitivity of 99.6% in the hospital-based survey and 94.6% in the community-based survey). Among four candidate questions on flexural dermatitis (Q1-2 to Q1-5), 'the folds of the elbow or behind the knees' was selected as major criteria due to Youden's index of >70%. Other 3 questions on flexural dermatitis, including around the neck, under the buttock, and around the wrist or ankle joints, were re-classified as minor criteria. 'Personal or family history of atopy' was also re-classified as minor criteria due to Youden's index of <70% (Table 3).
From the analysis on the results of the draft version of questionnaire, questions with a low sensitivity (<20% in any of hospital- or community-based surveys) were excluded from the final version of questionnaire. Among 5 candidate questions on non-flexural dermatitis (Q2-1 to Q2-5), 2 for hand/foot and nipple eczema were excluded from the minor criteria due to a low sensitivity (<20%) in a community-based survey. Among 5 candidate questions on environmental factors (Q3-1 to Q3-5), 3 aggravating factors (Q3-3 to Q3-5) were excluded from the minor criteria due to their low sensitivity (<20%) in a community-based survey (Table 3).
Questions on major criteria were finally narrowed down to 2 (Q1 and Q2), pertaining to 'recurrent skin rash in the last 12 months' (Q1) and 'itchy rash on the folds elbows or behind the knees' (Q2). Minor criteria were indexed by nine questions (Q3-Q11): one for atopy history (Q3), six for localized eczema, including three flexural dermatitis and three non-flexural dermatitis (Q4-Q9); and two for environmental factors (Q10 and Q11) (Table 2).
A total of 1,129 children from 2 different age groups of elementary school children (n=988, 7-12 years old) and preschool children (n=141, 4-6 years old) were enrolled in the survey as a derivation set. Following 2 skin examinations, 152 children were diagnosed with AD, yielding a standard AD prevalence rate of 13.5%. Non-AD children were allocated to the control group (n=977). Fulfillment of '2 major (Q1+Q2) (2M)' for 'eczema on the antecubtial/popliteal fossae' and '1 major (Q1) plus 4 or more minor (1M+4m)' for 'eczema on the non-antecubtial/popliteal fossae'were selected as optimal following our analysis of the derivation set (n=1,129 children); the estimated AD prevalence using our criteria was 13.4%, compared to 15.1% according to the ISAAC criteria. The diagnostic accuracy of the REACH ('2M' or '1M+4m') was also superior to that of the ISAAC criteria (sensitivity, 80.3% vs 73.0%; specificity, 97.0% vs 94.0%; PPV, 80.8% vs 65.3%; NPV, 96.9% vs 95.7%; and ER, 5.2% vs 8.9%) (Table 4).
We repeated our survey in a validation set (n=1,191) from 2 age groups of elementary schoolchildren (n=1,071, 7-12 years old) and preschool children (n=120, 4-6 years old). Following 2 skin examinations, 141 children were diagnosed with AD, yielding an AD prevalence of 11.8%. The non-AD children were allocated to the control group (n=1,050). In estimated AD prevalence, the REACH (12.3%, 95% CI, 10.5%-14.2%), which included 10.3% (n=123) for 'eczema on the antecubital/popliteal fossae' and 2.0% (n=24) for 'eczema on the non-antecubital/popliteal fossae, was closer to our gold standard than the ISAAC (14.4%, 95% CI, 12.4%-16.4%). The REACH criteria also exhibited improved diagnostic accuracy with fewer misclassifications (6.4%, n=76) compared to the ISAAC (10.0%, n=119; P<0.001) (Table 5). Taken together, we propose the REACH diagnostic criteria for AD, which included '2 major or more' for AD with eczema on the antecubital or popliteal fossae and '1 major and 4 minor or more' for AD without eczema on the antecubital or popliteal fossae (Table 6).
Heterogeneity in skin symptoms among AD patients renders the proposal of definitive diagnostic criteria problematic.215 The ISAAC criteria have been widely used as a standard diagnostic instrument for epidemiological surveys, but this system has exhibited variable validity when used in different ethnic groups and geographical settings.141718 As shown herein, overestimation of AD prevalence using inaccurate questionnaires has a potential to make a mistake in managing childhood AD in given communities or countries. We introduce the REACH as a new diagnostic instrument for childhood AD with improved outcomes in both the estimated AD prevalence and diagnostic accuracy, compared to the ISAAC.
Recurrent flexural dermatitis on the antecubital/popliteal fossae is an essential component for childhood AD in population-based surveys. Our results confirm that the antecubital/popliteal fossae represent the most sensitive area (sensitivity: 78.4% in hospital-based and 91.9% in community-based surveys) with respect to detection of recurrent flexural dermatitis in AD compared to other flexural areas. The 2 locations can be easily examined in a dressed status in summer. Stensen et al.19 suggested that the 2 locations are important for 'visible flexural dermatitis' in UK's criteria and can be amenable to examination while dressed.6 As flexural dermatitis only was not sufficient to detect all of the AD patients, we categorized AD into 'eczema on the antecubital/popliteal fossae' and 'eczema on the non-antecubital/popliteal fossae' to increase sensitivity of the REACH. The REACH was designed to detect not only the major group of AD with flexural dermatitis on the antecubital/popliteal fossae, but also the minor group of AD to manifest with eczema on the non-antecubital/popliteal fossae in combination with 'localized eczema' (6 questions) and/or 'environmental factors' (2 questions) and/or 'atopic history' (1 question). In our validation set, inclusion of 'eczema on the non-antecubital/popliteal fossae' improved the sensitivity from 66.7% to 75.2% (i.e., an 8.5% improvement) without changing the ER value (6.4%), compared to 'eczema on the antecubital/popliteal fossae' only. From this study, approximately 16% of childhood AD cases detected in community-based surveys were classified as the minor group of AD cases that do not have flexural dermatitis on the antecubital or popliteal fossae. We categorized a set of flexural and non-flexural dermatitis (except flexural dermatitis on the antecubital/popliteal fossae) as 'localized eczema' in the minor criteria of the REACH, in that they were highly heterogeneous among AD patients. The reduced incidence of misclassification that characterizes the REACH, compared to the ISAAC (P<0.001), might be due to the more in-depth questions that were included in the former instrument. In contrast with the ISAAC to inquire the locations of flexural dermatitis simply, questions for 'localized eczema' in the REACH were prepared in detail to describe acute or chronic nature of eczematous skin lesions, such as oozing, thickening, or postinflammatory hyperpigmentation. Furthermore, the REACH was designed to detect the minor group of AD by asking questions at atopy history and environmental factors.
Hanifin and Rajka's and KDA's criteria classified 'atopy history' in the major criteria as an important pathogenic factor of AD.316 In contrast, we classified 'atopy history' in the minor criteria due to its low specificity: a positive atopy history was observed in 50.6% and 60.6% of non-AD children in hospital- and community-based surveys, respectively. These findings are consistent with those of a Japanese study, in which positive atopy history among a non-AD control group was estimated at 44.4%.20
Ideal, worldwide-standardized tools for epidemiological surveys might be impossible to formulate due to the heterogeneity of genetic backgrounds and environmental factors among AD patients. Strictly, questionnaires on childhood AD for epidemiological surveys should be developed de novo for different countries.21 From this study, we propose the following components might be compulsory in preparing questionnaires for childhood AD. First, 'relapsing flexural dermatitis on the antecubital/popliteal fossae' is an essential component of the major criteria. Second, questions on 'localized eczema,' such as flexural and non-flexural dermatitis, should be included in questionnaires, as they are unambiguous, objective signs for children and their parents with a low recall bias. However, it is reasonable to classify them into the minor criteria due to high heterogeneity among AD patients. Third, 'atopy history' is an essential component for AD diagnosis as an important genetic factor in AD pathogenesis.22 However, questions on 'atopy history' are desirable to be classified in the minor criteria due to its low specificity. We should consider the possibility of recall bias or misdiagnosis in the 'atopy history' due to its broad spectrum including AD, asthma, and allergic rhinitis of whole family members (father, mother, and siblings). Fourth, 'environmental factors' as an important pathogenic factor of AD should be included in questionnaires. Interestingly, 'environmental factors' might be closely related with the host factors of AD. From the REACH, questions on 'unusually dry skin' and 'itch by sweating' are highly positive in both hospital- and community-based surveys in Korea. 'Unusually dry skin' can be regarded as an environmental factor aggravated in winter, which is closely related with the host factor of abnormal barrier function, including filaggrin mutation.2324 'Generalized dry skin' is frequently detected in most of the AD patients, regardless of their severity.25 'Itch by sweating' can be regarded as an environmental factor aggravated in summer, which is closely related with the host factor of abnormal neurogenic inflammation.2627 Many AD patients complained that they could not sweat well in hot and humid weather, suggesting the altered neuropathological pathways mediating pruritus as well as sweating in the skin of AD patients.28 The Schulz-Larsen questionnaire also contains 2 questions pertaining to these factors.2930 To our expectation, items for 'localized eczema' and 'environmental factors' can be modified in different countries with diverse genetic or environmental backgrounds.
We propose that the REACH, which is designed to detect both major and minor groups of AD, can be used as a novel diagnostic tool for epidemiological surveys of childhood AD. These questionnaire-based diagnostic criteria not only allow for accurate estimation of AD prevalence in a given population, but can also be used to provide valuable medical advice for participants. The REACH exhibits greater accuracy compared to the ISAAC, in terms of estimating AD prevalence and diagnostic accuracy, with significantly reduced instances of misclassification. Our study has limitations due to selection bias for the age and location. We selected 2 age groups, instead of whole-age groups for childhood AD, and 1 location, instead of the whole country. Further studies are required to validate the REACH on whole-age groups and the whole country, as well as in different countries or ethnicities in the context of large-scale, epidemiological surveys.
Figures and Tables
Table 1
Draft version of questionnaire for use as a preliminary survey in the hospital and community settings
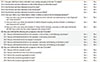
Table 2
The REACH (Reliable Estimation of Atopic dermatitis of ChildHood) questionnaire

Table 3
Diagnostic accuracy of candidate questions in the draft version of questionnaire (preliminary study conducted in hospital and community settings)

Table 4
Diagnostic accuracy of the REACH used in the derivation set (N=1,129)

*2M means recurrent eczema on the antecubital or/and popliteal fossae, and 1M means recurrent eczema on the other locations except the antecubital/popliteal fossae.
AD, atopic dermatitis; PPV, positive predictive value; NPV, negative predictive value; ER, error rate; M, major criteria; m, minor criteria.
Table 5
Diagnostic accuracy of the REACH and the ISAAC used in the validation set (N=1,191)

Notes
References
1. Brenninkmeijer EE, Schram ME, Leeflang MM, Bos JD, Spuls PI. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol. 2008; 158:754–765.
2. Deleuran M, Vestergaard C. Clinical heterogeneity and differential diagnosis of atopic dermatitis. Br J Dermatol. 2014; 170:Suppl 1. 2–6.
3. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; (92):44–47.
4. Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. working party's diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994; 131:406–416.
5. Williams HC, Burney PG, Pembroke AC, Hay RJ. U.K. Diagnostic Criteria for Atopic Dermatitis Working Party. Validation of the U.K. diagnostic criteria for atopic dermatitis in a population setting. Br J Dermatol. 1996; 135:12–17.
6. Williams HC, Forsdyke H, Boodoo G, Hay RJ, Burney PG. A protocol for recording the sign of flexural dermatitis in children. Br J Dermatol. 1995; 133:941–949.
7. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995; 8:483–491.
8. Beasley R. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998; 351:1225–1232.
9. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009; 124:1251–1258.e23.
10. Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long-term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol. 2006; 55:765–771.
11. Saeki H, Iizuka H, Mori Y, Akasaka T, Takagi H, Kitajima Y, et al. Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br J Dermatol. 2005; 152:110–114.
12. Kim DS, Lee JH, Lee KH, Lee MG. Prevalence and severity of atopic dermatitis in Jeju Island: a cross-sectional study of 4,028 Korean elementary schoolchildren by physical examination utilizing the three-item severity score. Acta Derm Venereol. 2012; 92:472–474.
13. Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015; 70:836–845.
14. Flohr C, Weinmayr G, Weiland SK, Addo-Yobo E, Annesi-Maesano I, Björkstén B, et al. How well do questionnaires perform compared with physical examination in detecting flexural eczema? Findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase two. Br J Dermatol. 2009; 161:846–853.
15. Pugliarello S, Cozzi A, Gisondi P, Girolomoni G. Phenotypes of atopic dermatitis. J Dtsch Dermatol Ges. 2011; 9:12–20.
16. Park YL, Kim HD, Kim KH, Kim MN, Kim JW, Ro YS, et al. Report from ADRG: a study on the diagnostic criteria of Korean atopic dermatitis. Korean J Dermatol. 2006; 44:659–663.
17. Choi WJ, Ko JY, Kim JW, Lee KH, Park CW, Kim KH, et al. Prevalence and risk factors for atopic dermatitis: a cross-sectional study of 6,453 Korean preschool children. Acta Derm Venereol. 2012; 92:467–471.
18. Haileamlak A, Lewis SA, Britton J, Venn AJ, Woldemariam D, Hubbard R, et al. Validation of the International Study of Asthma and Allergies in Children (ISAAC) and U.K. criteria for atopic eczema in Ethiopian children. Br J Dermatol. 2005; 152:735–741.
19. Stensen L, Thomsen SF, Backer V. Change in prevalence of atopic dermatitis between 1986 and 2001 among children. Allergy Asthma Proc. 2008; 29:392–396.
20. Saeki H, Iizuka H, Mori Y, Akasaka T, Takagi H, Kitajima Y, et al. Community validation of the U.K. diagnostic criteria for atopic dermatitis in Japanese elementary schoolchildren. J Dermatol Sci. 2007; 47:227–231.
21. Chalmers DA, Todd G, Saxe N, Milne JT, Tolosana S, Ngcelwane PN, et al. Validation of the U.K. Working Party diagnostic criteria for atopic eczema in a Xhosa-speaking African population. Br J Dermatol. 2007; 156:111–116.
22. Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015; 7:101–105.
23. Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008; 27:128–137.
24. Lee HJ, Lee SH. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res. 2014; 6:276–287.
25. Schario M, Lünnemann L, Stroux A, Reisshauer A, Zuberbier T, Blume-Peytavi U, et al. Children with dry skin and atopic predisposition: daily use of emollients in a participant-blinded, randomized, prospective trial. Skin Pharmacol Physiol. 2014; 27:208.
26. Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009; 53:48–54.
27. Papoiu AD, Wang H, Nattkemper L, Tey HL, Ishiuji Y, Chan YH, et al. A study of serum concentrations and dermal levels of NGF in atopic dermatitis and healthy subjects. Neuropeptides. 2011; 45:417–422.
28. Ständer S, Steinhoff M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp Dermatol. 2002; 11:12–24.
29. Schultz Larsen F, Diepgen T, Svensson Å. The occurrence of atopic dermatitis in North Europe: an international questionnaire study. J Am Acad Dermatol. 1996; 34:760–764.
30. Schultz Larsen F, Svensson Å, Diepgen TL, From E. The occurrence of atopic dermatitis in Greenland. Acta Derm Venereol. 2005; 85:140–143.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download