Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
Fig. 1
Protocol for the measurement of cytokines of α-GalCer-stimulated iNKT cells in FlaB-treated PBMC cultures. PBMCs were treated with FlaB for 48 hours. To stimulate iNKT cells, α-GalCer was added 24 hours before cytokine measurements. Secreted and intracellular cytokines were measured using ELISA and flow cytometry, respectively. In some experiments, IL-10R or IL-12 mAbs were added 6 hours before FlaB treatment to determine IL-10- or IL-12-dependent effect of FlaB on iNKT cell function.

Fig. 2
Representative examples of flow cytometric analysis. (A) Intracellular cytokines of iNKT cells in PBMCs. Lymphocytes and Vα24+ and Vβ11+ iNKT cells were gated. IL-4+, IFN-γ+ or IL-17+ cells were measured. Histograms show cytokine-positive iNKT cells (colored) and isotype antibodies (blank). (B) Appropriate measurement time point for each intracellular cytokine following α-GalCer stimulation in PBMCs. IL-4+, IFN-γ+, or IL-17+ iNKT cells were determined at baseline and 6, 12, and 24 hours after α-GalCer stimulation. Histograms show cytokine-positive iNKT cells (colored) and isotype antibodies (blank).

Fig. 3
IL-4, IFN-γ, and IL-17 production from α-GalCer-stimulated iNKT cells following FlaB treatment of PBMC cultures from asthma patients and healthy controls. FlaB treatment and α-GalCer stimulation were performed as described in Fig. 1. Cytokines in supernatants were measured by using ELISA. (A) Cytokine production in cells from asthma patients (n=10). (B) Cytokine production in cells from healthy controls (n=10). A Wilcoxon test was performed. Horizontal bars represent the median.

Fig. 4
Intracellular IL-4, IFN-γ, IL-17, and IL-10 production of α-GalCer-stimulated iNKT cells following FlaB treatment in PBMC cultures from asthma patients and healthy controls. FlaB treatment and α-GalCer stimulation were performed as described in Fig. 1. Intracellular cytokines were measured using flow cytometry. (A) Intracellular cytokine production in cells from asthma patients (n=10). (B) Intracellular cytokine production in cells from healthy controls (n=10). A Wilcoxon test was performed. Horizontal bars represent the median. NS, not significant.

Fig. 5
IL-10-dependent effect of FlaB on IL-4 and IL-17 production in iNKT cells from asthma patients (n=6) and IL-12-dependent effect of FlaB on IL-4 and IL-17 production in iNKT cells from normal controls (n=5). IL-10R or IL-12 mAbs were added before FlaB treatment as described in the Fig. 1 protocol. Secreted and intracellular cytokines were measured using ELISA (A) and flow cytometry (B) in cells from asthma patients, respectively, and using ELISA (C) and flow cytometry (D) in cells from normal controls, respectively. A Wilcoxon test was performed. Horizontal bars represent the median. NS, not significant.

Fig. 6
Effect of FlaB-treated DCs on intracellular IL-4 and IL-17 production of α-GalCer-stimulated iNKT cells following FlaB treatment of PBMCs from asthma patients. CD14+ monocyte-derived DCs were generated and treated for 48 hours. (A) IL-10 and IL-12 production from FlaB-treated DCs in asthma patients (n=4) and normal controls (n=3) measured by ELISA. In HDM-sensitive asthma patients (Table 2), FlaB-treated DCs and autologous CD3+ T cells were co-cultured with IL-10R or IL-12 mAbs in the presence of HDM extracts for 6 days. Intracellular cytokines of α-GalCer-stimulated iNKT cells were determined by flow cytometry. (B) Characteristics of each experimental group in the co-cultures. (C) IL-10-dependent inhibition by FlaB-treated DCs on IL-4+ iNKT cells (n=3). (D) IL-10-dependent inhibition by FlaB-treated DCs on IL-17+ iNKT cells (n=3). A Wilcoxon test was performed. Data represent the mean±SEM. For Fig. 6C and D, *P<0.05 and **P<0.01 compared to α-GalCer group. NS, not significant.

Fig. 7
IL-10-dependent induction of Foxp3+ iNKT cells following FlaB treatment of PBMCs from asthma patients. FlaB treatment and α-GalCer stimulation were performed as described in Fig. 1. IL-10R mAb was added before FlaB treatment, and intracellular cytokines were measured by flow cytometry. (A) A representative example for analyzing Foxp3+ iNKT cells. (B) IL-10-dependent induction of Foxp3+ iNKT cells (n=3). A Wilcoxon test was performed. Data represent the mean±SEM.

Table 1
Subject characteristics
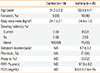
*P<0.01 compared to control by the Mann-Whitney U test. FEV1, forced expiratory volume in one second; †A subject was classified as atopic if any allergen caused a wheal 3 mm or larger in diameter than saline.
PC20, concentration of methacholine that provoked a 20% fall in FEV1.
NA, not applicable; ND, not determined.
Table 2
Characteristics of patients with HDM-sensitive asthma

M, male; F, female; FEV1, forced expiratory volume in one second; PC20, concentration of methacholine inducing 20% decrease in FEV1 from saline value; BDR, bronchodilator response expressed as % change in FEV1 at 20 minutes after inhaling 200 µg of salbutamol; SPT to Dermatophagoides farinae, skin prick to D. farinae expressed as longest diameter (mm×mm) and perpendicular diameter (mm×mm) at 20 minutes after the prick test; ImmunoCAP to D. farinae; serum D. farinae-specific IgE level by ImmunoCAP; ND, not determined.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download