Abstract
Papain is a proteolytic enzyme which is widely used in food industry, pharmaceuticals, and cosmetics. Occupational and non-occupational papain allergies have previously been documented; however, there are limited publications about papain allergy with its relative fruit allergy. Here, we present a case of occupational, IgE-mediated papain allergy with kiwi fruit and fig fruit allergy. A 53-year-old man suffered from rhinitis for several years, with the onset of his symptoms coinciding with the time he started to work at a sausage processing plant where papain is often used as a meat tenderizer. He began to experience symptoms of chest tightness, shortness of breath and wheezing shortly after starting work 5 years ago. Furthermore, he experienced several episodes of oral itching, and tongue and oropharyngeal angioedema after injestion of kiwi fruit and fig fruit. The patient had a lifelong history of allergic conjunctivitis, allergic rhinitis, and childhood asthma. Specific IgE was positive to kiwi fruit, papain and chymopapain (2.95 kUA/L, >100 kUA/L, and 95.0 kUA/L, respectively). Similar bands at 10-15 kDa in blotting with papain and kiwi fruit extracts were found. This patient showed a potential association between papain allergy and sensitization to kiwi fruit. We also reviewed 13 patients with papain allergy published in the literature, with 85% (11/13) of the patients sensitized through the respiratory tract, and 40% (4/11) having atopy. Further studies should focus on the determination of cross-reactive allergens between papain and its fruit relatives, and the prevalence of food allergy in patients with papain allergy should be investigated in a relatively large cohort.
Papain, a proteolytic enzyme extracted from the latex of the papaya tree and fruit (Carica papaya), has numerous uses and applications. It is not only widely used as a meat or cephalopod tenderizer and a beer clarificant in food industry, but it is also used for pharmaceuticals and cosmetics.1 IgE-mediated papain allergy in exposed workers was first reported in 1975.2 The prevalence of papain sensitization among exposed individuals from a relatively small cohort study was 41%.3 It has well documented that papain can induce reactions through different routes: respiratory,245 skin/conjunctival,678 or oral.9 Papain allergy can occur in occupational and non-occupational settings, and most of the affected subjects work in a factory,45 a biochemical laboratory,2 or a beauty salon.68 Food processors may affect IgE-mediated allergic occupational diseases through halation of airborne particles, skin contact, or ingestion. Daiana et al.10 reported occupational rhinoconjunctivitis due to maize in a snack processor. Here, we present a case of occupational, IgE-mediated papain allergy who works in a sausage processing factory.
A 53-year-old man was referred to our clinic with shortness of breath and wheezing. These symptoms improved following the use of salmeterol/flutieasone propionate on demand. The patient has a lifelong history of allergic conjunctivitis, allergic rhinitis, and childhood asthma. For 10 years, he worked in a sausage processing plant where papain was used as a meat tenderizer. The patient reported that his symptoms became more severe when he began to work at the sausage processing plant. He mentioned that some coworkers also had respiratory complaints as well. The patient experienced 2 episodes of oral itching, and angioedema of the oropharyngeal tract and tongue after eating kiwi fruit 8 years ago. He had similar symptoms within a few minutes after eating fig fruit 5 years ago. Physical examination was normal. Skin tests were positive to summer-autumn pollen I (++), spring pollen I (++), Fraxinus Americana (+) (Beijing Macro-Union Phamaceutical Co. Ltd., Beijing, China). The patient also exhibited positive skin responses to fresh kiwi fruit (+++) and papain (+++) (0.1 mg/mL). The total IgE level (ImmunoCAP, Phadia, Upsala, Sweden) was 214 kU/L, and specific IgE were positive to kiwi fruit (2.95 kUA/L), papain (>100 kUA/L),and chymopapain (95.0 kUA/L), but negative to latex, common silver birch, mountain juniper, common ragweed, mugwort, and scale len scale. Baseline spirometry results were normal.
Kiwi fruit and papain extracts were analyzed by SDS-PAGE as described by Laemmli. SDS-PAGE analysis of kiwi fruit extract showed multiple protein bands with an apparent molecular mass ranging from 10 to 30 kD, and papain protein showed some smear bands ranging from 10 to 35 kD (Figure A). As shown in Figure B, sera from the patient showed IgE binding to 21, 15, and 10 kD proteins in kiwi fruit extracts and papain proteins of 15 KD.
We present the 13 published papain allergy cases in Table. In general, most publications were based on isolated case reports and a small series of patients (Table). A death case due to papain inhalation was reported in 1977.4 Eighty-five percent (11/13) of the cases were sensitized through respiratory contact, and 40% (4/11) had atopy.
Papain-induced occupational asthma is a subtype of work-related asthma (WRA).11 Its clinical diagnosis was based on typical type I hypersensitivity symptoms on airway exposure to papain, clinical findings, papain positive skin testing, papain-specific IgE, and Western blotting assay.
Similar to previous reported cases,612 our case showed potential cross-reactivity between papain and kiwi fruit. Although kiwi fruit allergy in our patient was demonstrated by specific IgE levels and Western blotting data, our patient showed different binding patterns to kiwi fruit from published data, which reported actinidin (MW ≈30 kDa) to be the major allergen,13 differences in the recognition of allergens between populations of kiwi fruit-allergic patients from different countries partly due to genetic, dietary, or other environmental differences, and partly due to variations in kiwi fruit consumed. There are similar bands at 10-15 kDa in blotting with papain and kiwi fruit extracts, which may contribute to cross-reactivity. Further inhibition tests are needed to confirm our results.
There is possible cross-reactivity between papain and fig fruit according to the clinical history of our patient. Similarly, the cross-reactivity between papain and fig fruit has been documented by CAP inhibition, and the association between allergies to fig fruit and papain may be attributed to the presence of cross-reactive allergen structures.14
Previous studies suggested that papain allergy can occur in both atopic and non-atopic individuals as in our case with an atopic background. Baur et al.3 reported that although 14 out of the 33 workers had occupational exposure to papain, none of them had pre-existing allergic disease. However, Novey et al.17 demonstrated that atopic workers develop pulmonary symptoms and anti-papain antibodies significantly earlier after papain exposure than did others and that the concentration of papain, rather than the length of the exposure, is the most important factor for the development of clinical symptoms. Workers who have the greatest amount of dust exposure per workday have significantly more pulmonary symptoms.
Unlike several published studies,1216 our patient did not show cross-reactivity between papain and latex due to the absence of latex-specific IgE. Although published data suggested that the presence of cross-reactivity between papain and latex allergens due to IgE binding to some identical regions in papain and latex allergens, the difference may be due to papain IgE in this patient's recognition of other non-identical regions of papain.
Due to the high prevalence of sensitization in exposed workers and the potential profile of asthmatic life-threatening attacks, extensive protective measures should be taken to eliminate the risk of sensitization in the professional setting. Sensitized individuals should avoid exposure to both papain and its fruit relatives, e.g. kiwi fruit and fig fruit. Further studies should focus on the determination of cross-reactive allergens. The prevalence of relative fruit allergy in patients with papain allergy should be investigated in a relatively large cohort.
Figures and Tables
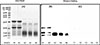 | FigureSDS-PAGE analysis and IgE immunoblotting in the patient and a healthy control.SDS-PAGE (A): lane M, molecular mass markers; Lane A1: kiwi fruit protein extract (KPE) 30 µg ; LaneA2: kiwi fruit protein extract 20 µg; lane A3: papain 30 µg; lane A4: papain 20 µg; laneA5: papain10 µg. Western blotting (B): lane M, molecular mass markers; lane B1-B5, immunblotting patterns of protein extracts incubated with the patient's serum, from B1 to B5, the proteins are KPE 30 µg, KPE 20 µg, papain 30 µg, papain 20 µg, and papain 10 µg, respectively. Western blotting (C): lane C1-C5, immunoblotting patterns of protein extracts incubated with the healthy control's serum, from C1 to C5, the loading proteins are KPE 30 µg, KPE 20 µg, papain 30 µg, papain 20 µg, and papain 10 µg, respectively. |
Table
Published cases of papain allergy until April 2, 2015 (in the English literature)

| Author (year) | No. | Age and gender | Sensitization routes | Occupational exposure | Papain application | clinical symptom | sIgE to papain | Skin test to papain | Challenge test | Atopy | Food sensitivity/allergy history |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Milne J et al. (1975)2 | 4 | Case 1: 27 yr, F | Respiratory | Yes (analytical chemist) | Proteolysis | A | NA | Positive | NA | No | No |
| Case 2: 32 yr, M | Respiratory | Yes (industrial research chemist) | Proteolysis | A | NA | Positive | NA | No | No | ||
| Case 3: 39 yr, M | Respiratory | Yes (factory worker) | Unknown | R, A, U | NA | NA | NA | No | No | ||
| Case 4: 24 yr, M | Respiratory | Yes (factory worker) | Unknown | Cough, tiredness | NA | NA | NA | No | No | ||
| Basingstoke Gazette* (1977)17 | 1 | 35 yr, M | Respiratory | Yes (factory worker) | Protein digestion | A | NA | NA | NA | Yes | No |
| Flindt ML et al. (1978)4 | 1 | 45 yr, M | Respiratory | Yes (factory worker) | Meat tenderizers | A | NA | Positive | NA | No | No |
| Novey HS et al. (1979)15 | 1 | 58 yr, M | Respiratory | Yes (factory worker) | Meat tenderizers | A | Positive | Positive | Bronchial challenge positive | NA | No |
| Mansfield LE et al. (1983)9 | 1 | 31 yr, M | Oral | No | Meat tenderizers | Anaphylaxis | NA | Positive | Oral challenge positive | Yes | No |
| Bernstein DI et al. (1984)7 | 1 | 31 yr, F | Ocular | No | Contact lens cleansing solution | C | Positive | Positive | Positive | Yes | No |
| Niinimaki A et al. (1993)8 | 1 | 30 yr, F | Ocular, respiratory | Yes (cosmetologist) | Abrasive cream | R,C | 52.5 KU/L | Positive | Nasal challenge positive | No | No |
| Soto-Mera MT et al. (2000)6 | 2 | Case 1: 20 yr, F | Skin, ocular, respiratory | Yes (beauty salon) | Removing adhesives | U, A, R, C | 47.3 kU/L | Positive | NA | No | Yes. Papaya |
| Case 2: 31 yr, F | Skin, ocular, respiratory | Yes (beauty salon) | Removing adhesives | R,A,U | 19.5 kU/L | Positive | NA | No | Yes. Kiwifruit, Papaya | ||
| Goeminne PC et al. (2013)5 | 1 | 52 yr, M | Respiratory | Yes (factory worker) | Unknown | A | >100 Ua/L | Positive | NA | No | No |
ACKNOWLEDGMENTS
We would like to thank Philip Mak, Department of Biology, Johns Hopkins University, for his great effort to revise our manuscript.
Notes
References
1. Amri E, Mamboya F. Papain, a plant enzyme of biological importance: a review. Am J Biochem Biotechnol. 2012; 8:99–104.
2. Milne J, Brand S. Occupational asthma after inhalation of dust of the proteolytic enzyme, papain. Br J Ind Med. 1975; 32:302–307.
3. Baur X, König G, Bencze K, Fruhmann G. Clinical symptoms and results of skin test, RAST and bronchial provocation test in thirty-three papain workers: evidence for strong immunogenic potency and clinically relevant 'proteolytic effects of airborne papain'. Clin Allergy. 1982; 12:9–17.
4. Flindt ML. Respiratory hazards from papain. Lancet. 1978; 1:430–432.
5. Goeminne PC, Adams E, Deschepper K, Valcke Y, Nemery B. Papain-induced asthma: a man with dyspnea from dawn till dust. Acta Clin Belg. 2013; 68:132–134.
6. Soto-Mera MT, López-Rico MR, Filgueira JF, Villamil E, Cidrás R. Occupational allergy to papain. Allergy. 2000; 55:983–984.
7. Bernstein DI, Gallagher JS, Grad M, Bernstein IL. Local ocular anaphylaxis to papain enzyme contained in a contact lens cleansing solution. J Allergy Clin Immunol. 1984; 74:258–260.
8. Niinimäki A, Reijula K, Pirilä T, Koistinen AM. Papain-induced allergic rhinoconjunctivitis in a cosmetologist. J Allergy Clin Immunol. 1993; 92:492–493.
9. Mansfield LE, Bowers CH. Systemic reaction to papain in a nonoccupational setting. J Allergy Clin Immunol. 1983; 71:371–374.
10. Guillen D, Barranco P, Palacín A, Quirce S. Occupational rhinoconjunctivitis due to maize in a snack processor: a cross-reactivity study between lipid transfer proteins from different cereals and peach. Allergy Asthma Immunol Res. 2014; 6:470–473.
11. Kwon SC, Song J, Kim YK, Calvert GM. Work-Related Asthma in Korea - findings from the Korea Work-Related Asthma Surveillance (KOWAS) program, 2004-2009. Allergy Asthma Immunol Res. 2015; 7:51–59.
12. Quarre JP, Lecomte J, Lauwers D, Gilbert P, Thiriaux J. Allergy to latex and papain. J Allergy Clin Immunol. 1995; 95:922.
13. Pastorello EA, Conti A, Pravettoni V, Farioli L, Rivolta F, Ansaloni R, et al. Identification of actinidin as the major allergen of kiwi fruit. J Allergy Clin Immunol. 1998; 101:531–537.
14. Díez-Gómez ML, Quirce S, Aragoneses E, Cuevas M. Asthma caused by Ficus benjamina latex: evidence of cross-reactivity with fig fruit and papain. Ann Allergy Asthma Immunol. 1998; 80:24–30.
15. Novey HS, Keenan WJ, Fairshter RD, Wells ID, Wilson AF, Culver BD. Pulmonary disease in workers exposed to papain: clinicophysiological and immunological studies. Clin Allergy. 1980; 10:721–731.
16. Baur X, Chen Z, Rozynek P, Düser M, Raulf-Heimsoth M. Cross-reacting IgE antibodies recognizing latex allergens, including Hev b 1, as well as papain. Allergy. 1995; 50:604–609.
17. Novey HS, Marchioli LE, Sokol WN, Wells ID. Papain-induced asthma--physiological and immunological features. J Allergy Clin Immunol. 1979; 63:98–103.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download