Abstract
Purpose
Pigment epithelium-derived factor (PEDF) is a recently discovered antiangiogenesis protein. PEDF possesses powerful anti-inflammatory, antioxidative, antiangiogenic, and antifibrosis properties. It has been reported that PEDF can regulate vascular endothelial growth factor (VEGF) expression. This study aimed to evaluate whether recombinant PEDF protein could attenuate allergic airway inflammation and airway remodeling via the negative regulation of VEGF using a murine model of chronic ovalbumin (OVA)-induced asthma and BEAS-2B human bronchial epithelial cells.
Methods
In an in vivo experiment, mice sensitized with OVA were chronically airway challenged with aerosolized 1% OVA solution for 8 weeks. Treated mice were given injections of recombinant PEDF protein (50 or 100 µg/kg body weight) via the tail vein. In an in vitro experiment, we investigated the effects of recombinant PEDF protein on VEGF release levels in BEAS-2B cells stimulated with IL-1β.
Results
Recombinant PEDF protein significantly inhibited eosinophilic airway inflammation, airway hyperresponsiveness, and airway remodeling, including goblet cell hyperplasia, subepithelial collagen deposition, and airway smooth muscle hypertrophy. In addition, recombinant PEDF protein suppressed the enhanced expression of VEGF protein in lung tissue and bronchoalveolar lavage fluid (BALF) in OVA-challenged chronically allergic mice. In the in vitro experiment, VEGF expression was increased after IL-1β stimulation. Pretreatment with 50 and 100 ng/mL of recombinant PEDF protein significantly attenuated the increase in VEGF release levels in a concentration-dependent manner in BEAS-2B cells stimulated by IL-1β.
Pigment epithelium-derived factor (PEDF) is a 50 kDa secreted glycoprotein with multiple functions, including anti-inflammation, antiangiogenic and antifibrosis properties.123 PEDF has been found to inhibit NF-ΚB activation and the expression of several proinflammatory genes such as intercellular cell adhesion molecule-1 (ICAM-1), tumor necrosis factor alpha (TNF-α) and matrix metalloproteinases.456 PEDF has also been shown to participate in the pathogenesis of allergic rhinitis.7 These functions suggest that PEDF may be a potential target in the prevention and treatment of allergic asthma.
Bronchial asthma is a chronic airway inflammatory disease associated with airway hyperresponsiveness (AHR) and airway remodeling. Airway remodeling refers to structural changes, including mucous gland hyperplasia, smooth muscle proliferation, subepithelial fibrosis, and vascular changes.89 Vascular endothelial growth factor (VEGF) is believed to be involved in the pathogenesis of allergic asthma, particularly airway remodeling.10 VEGF expression is regulated by several stimuli, including TNF-α, interleukin (IL)-1β, and lipopolysaccharides (LPS).11 Recent studies have shown that PEDF can negatively regulate VEGF expression.1213 We hypothesize that PEDF treatment may down-regulate VEGF expression and may lead to the inhibition of airway inflammation and airway remodeling.
The purpose of this study was to define the effects of PEDF on allergic airway inflammation and airway remodeling in BALB/c mice and in BEAS-2B human bronchial epithelial cells with in vivo and in vitro experiments, respectively. Our results clearly show that PEDF inhibits allergic airway inflammation and airway remodeling, at least in part, by suppressing VEGF expression.
Six- to eight-week-old female BALB/c mice (each weighing approximately 20 g) were purchased from Shanghai Laboratory Animal Inc. (Shanghai, China). All experimental animals were utilized under protocols approved by the Institutional Animal Care and Use Committee of Nanjing Medical University and the institutional animal ethics committee (Nanjing, China).
Thirty two mice were randomly divided into the control, OVA, PEDF low and PEDF high groups. On Days 0 and 14, the mice in the OVA, PEDF low, and PEDF high groups were immunized by intraperitoneal injection of 100 µg of chicken egg ovalbumin (OVA, Grade V; Sigma, St Louis, MO, USA) emulsified in 100 µL of aluminum hydroxide gel (InvivoGen, San Diego, CA, USA). On day 21, the mice were placed in a Plexiglas box (29×22×18 cm) and were airway challenged with 1% aerosolized OVA for 30 minutes per day, 3 days per week, for a period of 8 weeks. The mice in the PEDF low and high groups were given injections via the tail vein with 50 or 100 µg/kg body weight of recombinant PEDF protein13 (Peprotech, Rocky Hill, NJ, USA) before each OVA challenge. The mice in the control group received sensitization and airway challenge with phosphate-buffered saline (PBS) instead of OVA.
Airway responsiveness to acetylcholine chloride (ACh) was measured 24 hours after the last OVA challenge with an AniRes 2005 animal lung function analysis system (SYNOL High-Tech, Beijing, China) as previously described.16 Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (70 mg/kg). The trachea was then surgically exposed, and a plastic tube with an internal diameter of 4 mm was inserted into the trachea connected to a computer-controlled ventilator. A 27-gauge needle was inserted into the tail vein for ACh administration. The respiratory rate and the tidal volume were preset at 90 breaths/min and 6 mL/kg, respectively. Progressively increasing doses of ACh (10, 30, 90, and 270 µg/kg) were administered intravenously with a microinfusion pump (36 mL/min) via the caudalis vein. Data were obtained, and the maximum values of lung resistance (RL) were used to express changes in airway hyperreactivity.91011
After measurement of airway hyperreactivity, the retro-orbital puncture method was used to collect blood samples. Serum samples were collected after centrifuging at 1,000 g at 4℃ for 15 minutes, and plasma was stored at -70℃ until analysis. Airway lumina were washed 3 times with 0.5 mL volumes of saline. Bronchoalveolar lavage fluid (BALF) was centrifuged at 1,000 g at 4℃ for 15 minutes, and upper fluid samples were collected for detection by enzyme-linked immunosorbent assay (ELISA). The cell pellets were suspended for total inflammatory cell counts with a hemocytometer. The smears of BALF cells were stained with Wright's stain for differential cell counts. The cells in the BALF were counted by 2 independent investigators in a single-blind study analyzing at least 200 cells each from 4 different random locations using a microscope. Commercially available ELISA kits were used to evaluate the levels of the Th2 cytokines IL-4 (Jingmei Biotech, Shanghai, China), IL-5, IL-13, transforming growth factor-beta1(TGF-β1), and VEGF (R&D Systems, Minneapolis, MN, USA) in BALF and OVA-specific serum IgE (Shibayagi, Gunma, Japan).
The left lung was fixed in 4% paraformaldehyde overnight at 4℃ and then embedded in paraffin. Paraffin sections (5 µm) were stained with hematoxylin and eosin (H&E) to detect eosinophilic infiltration, periodic acid-Schiff (PAS) to assess mucus-secreting goblet cells, and Masson's trichrome stain to evaluate the peribronchiolar collagen layer or processed for α-smooth muscle actin (SMA) immunohistochemistry to estimate airway smooth muscle hypertrophy. Histological analyses were performed by pathologists blinded to the treatment groups. Scoring for mucus production was quantified as previously described15: 0, no goblet cells; 1, <25% of the epithelium; 2, 25%-50% of the epithelium; 3, 50%-75% of the epithelium; and 4, >75% of the epithelium. The area of peribronchial trichrome staining in the mouse lung is expressed as the area of trichrome staining per µm length of the basement membrane (BM) of bronchioles. At least 10 bronchioles with internal diameters of 150-200 µm in each slide were counted.
Lung sections were treated in xylene and rehydrated in graduated alcohol solutions. Endogenous peroxidase activity was blocked with 3% H2O2. Specimens were flooded with 5% normal goat serum to prevent the nonspecific absorption of immunoglobulin; then, specimens were incubated with anti-α-SMA monoclonal antibody (DAKO, Glostrup, Denmark) at a dilution of 1:50 for 90 minutes. A substitution of each primary antibody with PBS was used as a negative control. The slides were incubated overnight at 4℃, rinsed 3 times with PBS, and then incubated with peroxidase-labeled secondary antibody (DAKO, Glostrup, Denmark) for 30 minutes at room temperature. The sections were washed again with PBS, followed by diaminobenzidine staining. The sections were then counterstained with hematoxylin, dehydrated, and observed. Data are expressed as the area of α-SMA immunostaining per µm length of the BM of bronchioles with internal diameters of 150-200 µm.
A hydroxyproline assay was used to determine the total collagen content of the right lungs using a commercially available hydroxyproline detection kit (Nanjing Jiancheng Biotechnology, Nanjing, China). Briefly, frozen right lung tissues were homogenized in saline containing 0.1 mol/L phenylmethylsulfonyl fluoride. Then, the homogenized lung tissues were hydrolyzed in 6 mol/L HCl. The hydroxyproline concentration was measured as previously described.17
BEAS-2B human bronchial epithelial cells were cultured in 6-well round-bottom plates supplemented with 4 mM L-glutamine, 20 µg/mL streptomycin, 100 U/mL penicillin, and 10% fetal bovine serum (all from HyClone, Logan, UT, USA). After starvation, the cells were cultured with 500 ng/mL IL-1β (R&D Systems, Minneapolis, MN, USA) in the presence or absence of different doses of recombinant PEDF protein (50,100 ng/mL, Peprotech, USA).1819 The supernatants were collected after 48 hours, and VEGF levels were assayed using ELISA kits.
The right lung tissues from each group were washed with ice-cold PBS and homogenized, and the lysates were prepared in RIPA lysis buffer and centrifuged at 12,000 g at 4℃ for 15 minutes. Equal amounts (30 µg) of proteins were loaded onto 8% SDS-PAGE gels. Subsequently, the proteins were electrotransferred (100 V for 2 hours). The membrane was blocked for 1 hour with Tris-buffered saline containing 0.05% Tween 20 (TBST) plus 5% skim milk and then incubated with 1:1,000 anti-VEGF (Abcam, Cambridge, UK) or 1:1,000 anti-GAPDH (Cell Signaling Technology Inc., Beverly, MA, USA) overnight at 4℃. The membrane was washed 3 times with TBST and then incubated with HRP-conjugated goat antirabbit IgG for 1 hour at room temperature. The membrane was washed 3 times with TBST again and detected with an ECL detection system (Pierce Biotechnology, Rockford, IL, USA).
To determine the effects of PEDF on airway reactivity, lung resistance (LR) was evaluated in anesthetized mice by invasive whole-body plethysmography. There were no significant differences in baseline airway resistance between the 4 groups. The LR generated by the administration of Ach at doses from 30 to 270 µg/kg was dramatically increased in the OVA group compared to the control group. RL was significantly decreased in the recombinant PEDF treated group (50 or 100 µg/kg bodyweight) compared to the OVA group, implying that in vivo inflammation-mediated airway pathology was alleviated. Recombinant PEDF reduced AHR in a dose-dependent manner (Fig. 1A).
To determine the effects of PEDF on airway inflammation, the amount and classification of inflammatory cells in the BALF were evaluated. Few inflammatory cells were detected in saline-sensitized and saline-challenged control mice. The mice in the OVA group displayed a marked increase in the number of total inflammatory cells and eosinophils in the BALF. Treatment with PEDF significantly decreased the numbers of eosinophils and total inflammatory cells in BALF in a dose-dependent manner (P<0.05, Fig. 1B). In addition, HE staining showed a marked reduction in the number of total inflammatory cells around the airway and the blood vessels in PEDF-treated mice compared to the OVA group (Fig. 2).
To determine the effects of PEDF on Th2 cytokines and TGF-β1, we measured the levels of T-helper type 2 (Th2) cytokines, such as IL-4, IL-5, IL-13, and TGF-β1 in BALF. IL-4, IL-5, IL-13, and TGF-β1 in BALF were dramatically increased in the OVA group compared to the control group (P<0.05, Fig. 3). Administration of PEDF significantly decreased Th2 cytokine and TGF-β1 levels in BALF in a dose dependent manner compared to the OVA group (P<0.05, Fig. 3).
To evaluate the effect of PEDF on IgE in serum, we also measured the level of OVA-specific IgE in serum. OVA-specific IgE in serum was obviously enhanced in the OVA group compared to the control group (P<0.05, Fig. 3), treatment with PEDF significantly inhibited the level of OVA-specific IgE in a dose dependent manner (P<0.05, Fig. 3).
To determine the effects of PEDF on airway remodeling changes, we determined the effects of PEDF on goblet cell metaplasia, smooth muscle proliferation, and subepithelial fibrosis. PAS staining was used to identify mucus-containing cells in the airway epithelium (Fig. 4A-D). Significant goblet cell hyperplasia and mucus hypersecretion were found in the OVA mice compared to the control group (P<0.05, Fig. 4E). This increase was evidently inhibited in mice treated with PEDF. Furthermore, PEDF reduced the number of goblet cells in the lumen of the bronchioles in a dose-dependent manner.
Masson's trichrome staining revealed dense collagen deposition/sub-epithelial fibrosis throughout the interstitium surrounding the airways and vessels of lung tissues in the OVA group compared to the control group (Fig. 5A-D). PEDF treatment obviously reduced collagen deposition compared to the OVA group (P<0.05, Fig. 5E).
Total lung hydroxyproline content was significantly increased in the OVA group compared to the control mice (P<0.05, Fig. 5F). In contrast, treatment with PEDF resulted in a significant reduction in total lung hydroxyproline content compared to the OVA group.
Additionally, α-SMA immunostaining was used to evaluate the thickness of airway smooth muscle. A marked increase in the α-SMA-staining areas in the peribronchiolar region was found in the OVA mice compared with the control mice (Fig. 6A-D). Administration of PEDF significantly reduced the area of the α-SMA-stained smooth muscle layer in a dose-dependent manner compared to the OVA group (P<0.05, Fig. 6E).
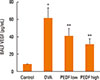
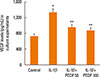
To determine whether VEGF is inhibited by PEDF in this model, we evaluated VEGF protein expression in mouse pulmonary tissue and VEGF levels in BALF. Compared to the control group, the OVA group had higher VEGF protein levels in the pulmonary tissue, and PEDF treatment prevented this increase (P<0.05, Fig. 7). ELISA also showed a marked increase in BALF VEGF levels in OVA-challenged mice. The mice treated with PEDF demonstrated lower levels of VEGF in BALF compared to the OVA group (Fig. 8). We also observed that PEDF administration reduced VEGF in a dose-dependent manner in the BALF and lung tissue of OVA-challenged mice.
VEGF released from airway epithelial cells can aggravate airway inflammation and airway remodeling. To further ascertain the anti-inflammatory and antiremodeling mechanisms of PEDF, we studied the effects of PEDF on IL-1β-induced VEGF expression in BEAS-2B cells. The levels of VEGF in culture supernatants from BEAS-2B cells induced by IL-1β were significantly increased compared to unstimulated cells. PEDF evidently inhibited the release of VEGF from BEAS-2B cells stimulated with IL-1β in a dose-dependent manner (P<0.05, Fig. 9).
Airway remodeling is an important pathophysiological characteristic of chronic asthma and correlates with the duration and severity of asthma.8 In this study, we evaluated the effects of recombinant PEDF on airway remodeling in chronic allergic asthma. Our results showed that recombinant PEDF had significant inhibitory effects on eosinophilic airway inflammation, AHR, and airway remodeling in this asthma model. In addition, treatment with recombinant PEDF resulted in marked inhibitory effects on the high expression of VEGF in lung tissues of OVA-challenged mice. Furthermore, recombinant PEDF administration suppressed VEGF release from BEAS-2B cells stimulated with IL-1β.
Th2-mediated airway inflammation present within the lung is fundamental in asthma. The Th2 cytokines IL-4, IL-5, and IL-13 are considered the key molecular mechanisms of asthma and have been shown to be associated with airway inflammation.2021222324 In the present study, the production of Th2 cytokines was significantly increased in OVA-challenged mice. Treatment with PEDF inhibited the levels of Th2 cytokines, such as IL-4, IL-5, and IL-13 in BALF, compared to OVA-challenged mice. In addition, recombinant PEDF prevented an increase in total inflammatory cells, especially eosinophils. Lung histological analysis also demonstrated that recombinant PEDF suppressed inflammatory cell infiltration, mucus hypersecretion, and goblet cell metaplasia in the airway. The results indicated that recombinant PEDF exerts significant anti-inflammation effects in chronic OVA-induced allergic mice.
Collagen deposition/subepithelial fibrosis in the airways, an important aspect of airway remodeling, is related to clinically severe asthma and a decline in pulmonary function.25 Previous studies showed that PEDF possesses antifibrosis properties exerted via inhibition of the TGF-β1 expression.2627 TGF-β1, a pro-fibrotic cytokine, leads to subepithelial collagen and extracellular matrix protein deposition in asthma.28 Thus, a marked increase in collagen deposition was observed around the airway in chronic OVA-induced mice. We show for the first time that the administration of recombinant PEDF not only significantly inhibits the TGF-β1 level in BALF in ova-induced mice, but also prevents subepithelial collagen deposition, as determined by Masson's trichrome staining and hydroxyproline analyses in chronic allergic asthma.
Airway smooth muscle hyperplasia is another key characteristic of airway remodeling and appears to be attributable to exaggerated AHR in asthma.29 In this study, the α-SMA stained area was significantly increased in chronic OVA-exposed mice. The chronic OVA-induced increase in airway smooth muscle was significantly inhibited by recombinant PEDF administration. The ability of recombinant PEDF to suppress airway smooth muscle thickness may be an important reason for its suppression of AHR.
VEGF is a potent stimulator of angiogenesis and structural changes in asthma.30 VEGF can aggravate Th2-type airway inflammation via IL-13-dependent and -independent mechanisms.31 Epithelial cell-derived VEGF promotes airway remodeling in asthma.32 VEGF levels are increased in sputum and lung tissues from asthmatic patients and correlate with the disease severity of asthma.333435 The inhibition of VEGF can lead to a significant reduction in basement membrane thickness and goblet cell hyperplasia.36 Previous studies showed that PEDF counteracts the biological actions of VEGF via down-regulating VEGF expression or binding VEGF receptors.3738 The results of our study showed that expression of the VEGF protein was enhanced after chronic OVA challenge, and treatment with recombinant PEDF decreased VEGF protein expression in the lung tissues and VEGF levels in the BALF of chronic OVA-induced allergic mice. In addition, recombinant PEDF significantly suppressed VEGF release from BEAS-2B cells stimulated by IL-1β. Therefore, we hypothesized that recombinant PEDF may inhibit airway inflammation and airway remodeling by reducting the expression of VEGF in a chronic asthma model.
In conclusion, our data demonstrated that PEDF exerts significant anti-inflammatory and antiremodeling effects in a chronic allergic asthma model. In addition, recombinant PEDF obviously inhibited VEGF expression in vivo and in vitro. Our findings indicate that PEDF might be a therapeutic target for chronic allergic asthma.
Figures and Tables
 | Fig. 1PEDF suppresses (A) airway hyperreactivity (AHR) and (B) inflammatory cell accumulation in bronchoalveolar lavage fluid (BALF) in a murine model of asthma. (A) PEDF decreased AHR compared to ovalbumin (OVA)-sensitized and challenged mice. (B) PEDF decreased the accumulation of total inflammatory cells, as well as eosinophils, compared to the OVA group. Data are presented as means±SEM (n=8 per group). *P<0.05 compared to the control group; **P<0.05 compared to the OVA group. |
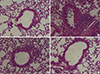 | Fig. 2PEDF attenuates airway inflammation in a murine model of asthma. Hematoxylin and eosin staining (original magnification ×100): (A) control group, (B) OVA group, (C) PEDF low group, and (D) PEDF high group. |
 | Fig. 3PEDF inhibits IL-4, IL-5, IL-13, and TGF-β1 in BALF and OVA-specific IgE in serum in a murine model of asthma. Data are presented as means±SEM (n=8 per group). *P<0.05 compared to the control group; **P<0.05 compared to the OVA group. |
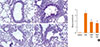 | Fig. 4PEDF attenuates airway goblet cell hyperplasia and mucus production in a murine model of asthma. Periodic acid-Schiff (PAS) staining (original magnification ×200): (A) control group, (B) OVA group, (C) PEDF low group, and (D) PEDF high group. (E) Quantitative analyses of mucus production in lung sections were performed as described in the Methods. Data are presented as means±SEM (n=8 per group). *P<0.05 compared to the control group; **P<0.05 compared to the OVA group. |
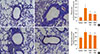 | Fig. 5PEDF inhibits peribronchiolar fibrosis in chronic experimental asthma. Masson's trichrome-positive peribronchiolar collagen layer (original magnification ×200) of lung tissue from (A) control group, (B) OVA group, (C) PEDFlow group, and (D) PEDFhigh group. (E) Quantitative analyses of the mean area of airway fibrosis and (F) total lung hydroxyproline content were determined as described in the Methods. Data are presented as means±SEM (n=8 per group). *P<0.05 compared to the control group; **P<0.05 compared to the OVA group. |
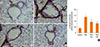 | Fig. 6PEDF inhibits the areas of α-smooth muscle actin (SMA) in a murine model of asthma. Peribronchial α-SMA immunostaining (immunohistochemistry, original magnification ×200) of lung tissue from (A) control group, (B) OVA group, (C) PEDF low group, and (D) PEDF high group. (E) Quantitative analyses of immunostaining area for peribronchial α-SMA. Data are presented as means±SEM (n=8 per group). *P<0.05 compared to the control group; **P<0.05 compared to the OVA group. |
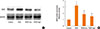 | Fig. 7PEDF ameliorates the expression of VEGF protein in lung tissue in chronic experimental asthma. (A) Total protein from lung tissue was extracted 24 hours after the final OVA challenge and subjected to Western blot analysis of VEGF. GAPDH was utilized as the standard control. (B) The band signal strength of VEGF expressed as a ratio to GAPDH. Data are presented as means±SEM (n=8 per group). *P<0.05 compared to the control group; **P<0.05 compared to the OVA group. |
Notes
References
1. Awad AS, Gao T, Gvritishvili A, You H, Liu Y, Cooper TK, et al. Protective role of small pigment epithelium-derived factor (PEDF) peptide in diabetic renal injury. Am J Physiol Renal Physiol. 2013; 305:F891–F900.
2. Tsai TH, Shih SC, Ho TC, Ma HI, Liu MY, Chen SL, et al. Pigment epithelium-derived factor 34-mer peptide prevents liver fibrosis and hepatic stellate cell activation through down-regulation of the PDGF receptor. PLoS One. 2014; 9:e95443.
3. Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006; 20:323–325.
4. Liu X, Chen HH, Zhang LW. Potential therapeutic effects of pigment epithelium-derived factor for treatment of diabetic retinopathy. Int J Ophthalmol. 2013; 6:221–227.
5. Ide Y, Matsui T, Ishibashi Y, Takeuchi M, Yamagishi S. Pigment epithelium-derived factor inhibits advanced glycation end product-elicited mesangial cell damage by blocking NF-kappaB activation. Microvasc Res. 2010; 80:227–232.
6. Alcantara MB, Dass CR. Pigment epithelium-derived factor as a natural matrix metalloproteinase inhibitor: a comparison with classical matrix metalloproteinase inhibitors used for cancer treatment. J Pharm Pharmacol. 2014; 66:895–902.
7. Kang HJ, Park HH, Chae SW, Hwang SJ, Lee SH, Lee SH, et al. Increased expression of pigment epithelium-derived factor in allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2008; 134:1094–1098.
8. Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011; 128:451–462.
9. Paik SH, Kim WK, Park JS, Park CS, Jin GY. A quantitative study of airway changes on micro-CT in a mouse asthma model: comparison with histopathological findings. Allergy Asthma Immunol Res. 2014; 6:75–82.
10. Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007; 19:676–680.
11. Koyama S, Sato E, Tsukadaira A, Haniuda M, Numanami H, Kurai M, et al. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J. 2002; 20:1449–1456.
12. Chuderland D, Ben-Ami I, Bar-Joseph H, Shalgi R. Role of pigment epithelium-derived factor in the reproductive system. Reproduction. 2014; 148:R53–R61.
13. Seki R, Yamagishi S, Matsui T, Yoshida T, Torimura T, Ueno T, et al. Pigment epithelium-derived factor (PEDF) inhibits survival and proliferation of VEGF-exposed multiple myeloma cells through its anti-oxidative properties. Biochem Biophys Res Commun. 2013; 431:693–697.
14. Ueda S, Yamagishi S, Matsui T, Jinnouchi Y, Imaizumi T. Administration of pigment epithelium-derived factor inhibits left ventricular remodeling and improves cardiac function in rats with acute myocardial infarction. Am J Pathol. 2011; 178:591–598.
15. Zha WJ, Qian Y, Shen Y, Du Q, Chen FF, Wu ZZ, et al. Galangin abrogates ovalbumin-induced airway inflammation via negative regulation of NF-κB. Evid Based Complement Alternat Med. 2013; 2013:767689.
16. Du Q, Feng GZ, Shen L, Cui J, Cai JK. Paeonol attenuates airway inflammation and hyperresponsiveness in a murine model of ovalbumin-induced asthma. Can J Physiol Pharmacol. 2010; 88:1010–1016.
17. Du Q, Zhou LF, Chen Z, Gu XY, Huang M, Yin KS. Imiquimod, a toll-like receptor 7 ligand, inhibits airway remodelling in a murine model of chronic asthma. Clin Exp Pharmacol Physiol. 2009; 36:43–48.
18. Yang J, Chen S, Huang X, Han J, Wang Q, Shi D, et al. Growth suppression of cervical carcinoma by pigment epithelium-derived factor via anti-angiogenesis. Cancer Biol Ther. 2010; 9:967–974.
19. Rogers ME, Navarro ID, Perkumas KM, Niere SM, Allingham RR, Crosson CE, et al. Pigment epithelium-derived factor decreases outflow facility. Invest Ophthalmol Vis Sci. 2013; 54:6655–6661.
20. Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994; 24:73–80.
21. Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001; 167:4668–4675.
22. Mishra A, Weaver TE, Beck DC, Rothenberg ME. Interleukin-5-mediated allergic airway inflammation inhibits the human surfactant protein C promoter in transgenic mice. J Biol Chem. 2001; 276:8453–8459.
23. Liou CJ, Cheng PY, Huang WC, Chan CC, Chen MC, Kuo ML, et al. Oral lovastatin attenuates airway inflammation and mucus secretion in ovalbumin-induced murine model of asthma. Allergy Asthma Immunol Res. 2014; 6:548–557.
24. Kim TH, Park YM, Ryu SW, Kim DJ, Park JH, Park JH. Receptor interacting protein 2 (RIP2) is dispensable for OVA-induced airway inflammation in mice. Allergy Asthma Immunol Res. 2014; 6:163–168.
25. Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998; 102:783–788.
26. Mao T, Gao L, Li H, Li J. Pigment epithelium-derived factor inhibits high glucose induced oxidative stress and fibrosis of cultured human glomerular mesangial cells. Saudi Med J. 2011; 32:769–777.
27. Schmitz JC, Protiva P, Gattu AK, Utsumi T, Iwakiri Y, Neto AG, et al. Pigment epithelium-derived factor regulates early pancreatic fibrotic responses and suppresses the profibrotic cytokine thrombospondin-1. Am J Pathol. 2011; 179:2990–2999.
28. Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol. 2007; 85:348–356.
29. Martin JG, Duguet A, Eidelman DH. The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease. Eur Respir J. 2000; 16:349–354.
30. Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep. 2013; 13:1–9.
31. Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004; 10:1095–1103.
32. Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012; 129:990.e6–997.e6.
33. Asai K, Kanazawa H, Kamoi H, Shiraishi S, Hirata K, Yoshikawa J. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clin Exp Allergy. 2003; 33:595–599.
34. Chetta A, Zanini A, Foresi A, D'Ippolito R, Tipa A, Castagnaro A, et al. Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy. 2005; 35:1437–1442.
35. Zou H, Fang QH, Ma YM, Wang XY. Analysis of growth factors in serum and induced sputum from patients with asthma. Exp Ther Med. 2014; 8:573–578.
36. Yuksel H, Yilmaz O, Karaman M, Bagriyanik HA, Firinci F, Kiray M, et al. Role of vascular endothelial growth factor antagonism on airway remodeling in asthma. Ann Allergy Asthma Immunol. 2013; 110:150–155.
37. Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006; 37:1–12.
38. Johnston EK, Francis MK, Knepper JE. Recombinant pigment epithelium-derived factor PEDF binds vascular endothelial growth factor receptors 1 and 2. In Vitro Cell Dev Biol Anim. 2015; 51:730–738.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download