Abstract
Purpose
The purpose of this study was to establish the diagnostic decision point (DDP) of peanut specific IgE (sIgE) for predicting the outcome of oral food challenge (OFC). We also evaluated the usefulness of sIgE to peanut components (Ara h 1, 2, 3, 8, and 9) in diagnosing peanut allergy.
Methods
Korean children aged over 12 months with a suspected peanut allergy were enrolled. Diagnosis of peanut allergy was confirmed by an open OFC or through the convincing history of anaphylaxis. Cutoff levels of sIgE to peanut and peanut components were determined by analyzing receiver operating characteristic curves.
Results
Forty-eight children (22 boys and 26 girls) with a suspected peanut allergy were enrolled. The previously established DDP for peanut-sIgE antibodies (14 kU/L) showed a sensitivity of 22.7%, specificity of 100%, positive predictive value (PPV) of 100%, and negative predictive value of 60.4% in our study population. The median levels of peanut-sIgE (5.4 kU/L vs 1.1 kU/L, P<0.001) and Ara h 2-sIgE (0.8 kU/L vs 0 kU/L, P<0.001) were significantly higher in the peanut allergy group than in the peanut tolerance group. The peanut-sIgE concentration indicating a PPV of 100% was 10.3 kU/L. The Ara h 2-sIgE level of 4.0 kU/L had a PPV of 100%.
Peanuts are one of the most common food allergens, although the prevalence of peanut allergy varies according to region and race.1234 The estimated prevalence of peanut allergy ranges from 0.4 to 1.8% and seems to be increasing in Western countries.567 However, the prevalence of peanut allergy in Asian countries has been reported to be lower than Western countries.34 The parent-reported incidence of peanut allergy in Korea is 0.68% in infants and 0.34% in early childhood.89 Nevertheless, a great deal of attention has been focused on peanut allergy, because it is often severe and has a tendency to be lifelong.10
Although the oral food challenge (OFC) is considered the gold standard test to confirm food allergy, predictive diagnostic decision point (DDP) values for specific IgE (sIgE) antibodies have been determined and are widely used in clinical settings to avoid an unnecessary OFC.1112 A recent Korean study demonstrated much higher DDP levels of sIgE for the diagnosis of egg allergy or cow's milk allergy than the DDPs previously established in other countries.13 This could be due to differences in the study population, such as race and region, as well as the prevalence of food allergy.1314 It indicates that the DDP for peanut allergy must be evaluated in each region, although the cutoff values were previously reported to be 14 kU/L.1112
Ara h 1, 2, and 3 are heat-stable seed storage proteins that are well defined major peanut allergens.7 Among them, Ara h 2 is the most frequent and important peanut allergen in Western children.7151617 A study of Korean children demonstrated that Ara h 2 is associated with severe clinical symptoms, but is a less prevalent peanut allergen than in Western subjects.18 However, the utility of component resolved diagnostics (CRD) for the diagnosis of peanut allergy was not investigated in Korea.
In the present study, we attempted to validate the previously established DDP for predicting the outcome of OFCs in Korean children and to find optimal cutoff levels of peanut-sIgE antibodies for the diagnosis of peanut allergy. In addition, we evaluated the usefulness of sIgE antibodies to peanut components such as Ara h 1, 2, 3, 8, and 9 for discrimination of clinically relevant peanut allergy.
We enrolled children ≥12 months of age who visited Samsung Medical Center and Pusan National University Hospital for evaluation of suspected allergy to peanuts from January 2011 to December 2013. The diagnosis for peanut allergy was confirmed by an open OFC. Children who had a definitive history of anaphylaxis within a year did not undergo an OFC, but were included in the statistical analysis. A patient was assigned to the peanut-allergy group when (1) the patient or his/her guardians reported a convincing history of anaphylaxis after peanut ingestion and had detectable peanut sIgE, or (2) when a patient had a positive OFC result. A patient was assigned to the peanut-tolerance group based on a negative OFC result. Anaphylaxis was defined according to the World Allergy Organization Anaphylaxis Guidelines.19 The study protocol was approved by the Institutional Review Board of Samsung Medical Center and Pusan National University Hospital.
Serum samples were analyzed using immunoCAP (Thermo Fisher Scientific Inc., Waltham, MA, USA) to quantify total IgE and sIgE antibodies to peanuts, Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9. Sensitization was defined when the sIgE levels was 0.35 kU/L or greater. Levels of sIgE antibodies above 100 kU/L were assigned a value of 101 kU/L for analysis. The six samples for this study were provided by Pusan National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. Six samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Open OFCs were performed under the supervision of allergists according to the Korean guidelines.20 Peanut kernels were roasted at 170℃ for 10 minutes. Starting dosage was 0.1 g and was increased every 15 minutes until a total dose of 10 g was reached or until the patient reported allergic symptoms. A positive test result was confirmed by the attending pediatric allergist when one or more of the following symptoms developed within 2 hours of the last challenge dose: urticaria, angioedema, rhinorrhea, cough, wheezing, stridor, dyspnea, vomiting, or hypotension.202122
Statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC, USA) and the R software package, version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). The median level of sIgE (kU/L) and interquartile range (IQR) were calculated. Mann-Whitney U and Fisher exact tests were used to compare continuous and binary variables between the peanut allergy and tolerance groups, respectively. Performance characteristics such as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were analyzed by using the previously established DDP of 14 kU/L as the peanut-sIgE level.1112 Receiver operating characteristic (ROC) curves were constructed using the ROCR R package. The optimal cutoffs were determined by maximizing the PPV. A P value <0.05 was considered significant.
Overall, 48 children (22 boys and 26 girls) with a suspected peanut allergy were enrolled. Among them, five children had a definitive history of anaphylaxis after the ingestion of peanuts (Fig. 1). The mean age of the study subjects was 4.9±2.9 years (range, 1.0-14.0 years). Of 48 children, five (10.4%) were younger than 24 months, with 14 children (29.2%) between 24 and 47 months of age, 15 children (31.2%) between 48 to 71 months, and 14 children (29.2%) above the age of 72 months. Concomitant allergic diseases were found in 40 (83.3%) subjects, with 81.3% having atopic dermatitis, 22.9% having asthma, and 29.2% having allergic rhinitis. Twenty-two children was confirmed to have peanut allergy, while 26 passed the peanut challenge and classified into peanut-tolerance group. In children with peanut allergy, the most frequent symptoms were cutaneous manifestations (100%), followed by respiratory symptoms (27.3%), gastrointestinal symptoms (13.6%), and cardiovascular symptoms (4.5%). Overall, 31.8% of the reactions involved two or more systems. Characteristics and total IgE levels were not different between the peanut-allergy group and peanut-tolerance group (Table 1).
Of 48 children, 38 (79.2%) were sensitized to peanuts. Their median level of peanut-sIgE was 2.6 kU/L (range 0-101 kU/L). The proportion of children sensitized to peanut was significantly higher in the peanut-allergy group than in the peanut-tolerance group (P= 0.013). The median level of peanut-sIgE was also significantly higher in the peanut-allergy group than in the peanut-tolerance group (5.4 vs 1.1 kU/L, P<0.001) (Fig. 2). The median level of Ara h 2-sIgE antibodies was also higher in the peanut-allergy group than in peanut-tolerance group (0.8 vs 0 kU/L, P< 0.001) (Fig. 2). More children were sensitized to Ara h 2 in the peanut-allergy group than in the peanut-tolerance group (P<0.001). However, there were no differences in the proportion of children with sIgE antibodies to Ara h 1, 3, 8, and 9 or the median levels of sIgE antibodies between allergy and tolerance groups.
When we applied the previously established DDP for peanutsIgE antibodies to the study population, the sensitivity, specificity, PPV, and NPV values obtained were 22.7%, 100%, 100%, and 60.4%, respectively. The relationship between sensitivity and specificity was further evaluated by analysis of the ROC curve, which showed an acceptable area under the curve (AUC) of 0.91 (Fig. 3). The peanut-sIgE concentration indicating a 100% risk of reaction was 10.3 kU/L (sensitivity 31.8%, specificity 100%, PPV 100% NPV 63.4%) (Table 2). Peanut-sIgE levels of 0.7 kU/L had a NPV of 90.1%, PPV of 56.8%, sensitivity of 95.4%, and specificity of 38.4%. The ROC curve analysis for Ara h 2-sIgE antibodies yielded an acceptable AUC of 0.82 (Fig. 3). The Ara h 2-sIgE level indicating a 100% risk of reaction was 4.0 kU/L (sensitivity 31.8%, specificity 100%, PPV 100%, NPV 63.4%) (Table 2). Ara h 2-sIgE level of 0.2 kU/L had a NPV of 77.8%, PPV of 76.2%, sensitivity of 72.7%, and specificity of 80.8%.
Peanut-sIgE level can be used by the physician to predict the outcomes of OFCs in patients suspected of having peanut allergy; the reported cutoff value is 14 kU/L.1112 Our data showed that the peanut-sIgE concentration indicating a 100% risk of reaction was 10.3 kU/L. The previously established DDP of peanut-sIgE level was also a useful predictor for the outcomes of OFCs in Korean children suspected of peanut allergy, because the values for obtained PPV and specificity were all 100%. These levels are in close agreement with those of previous studies in various countries. For example, a previous US study reported that 15 kU/L was the 100% DDP for peanuts, while an Australian study reported that 10 kU/L was the 100% DDP for peanuts.1423 It is noteworthy that the level of peanut-sIgE for the prediction of OFC is similar among these study populations despite differences in race, age ranges, and dietary habits. A large prospective study is needed to confirm the reliability and accuracy of our data.
We demonstrated that sensitization to Ara h 2 was more frequent than other peanut components, and was associated with clinical peanut allergy. There is a discrepancy among countries regarding sIgE reactivity to components other than Ara h 2. For example, sIgE to Ara h 9 is prominent in peanut allergic patients in Mediterranean studies, while Ara h 2 and Ara h 6 are the most frequently recognized major peanut allergens in Dutch children242526 A Singaporean study reported that 87% of patients reacted to Ara h 1 and 2 and 55% to Ara h 3.27 The characteristics of the study population and dietary habits could have an influence on the frequency of sensitization to peanut components.18
Several previous CRD-based studies have shown that Ara h 2 is the most important allergen for discriminating between peanut allergy and tolerance.71518282930 We also found that median levels of sIgE to Ara h 2 were significantly higher in the peanutallergy group than in the peanut-tolerance group. The AUC of sIgE to Ara h 2 was 0.82, which was better than the AUCs of sIgE to Ara h 1, 3, 8, and 9. The Ara h 2-sIgE level indicating a 100% risk of allergic reactions was 4.0 kU/L. In an Australian study, it has been reported that 1.92 kU/L of Ara h 2-sIgE had the 96% PPV for peanut allergy,29 while a Dutch study revealed 5 kU/L and 10 kU/L as the 96% and 100% PPV, respectively.15 Ara h 2-sIgE test has, therefore, been proposed to provide strong predictive accuracy for the diagnosis of peanut allergy.729 However, we found that the AUC for peanut-sIgE was greater than that for Ara h 2-sIgE, although their performance characteristics were similar. It indicates that the measurement of Ara h2-sIgE level does not provide additional benefit in predicting outcomes of OFCs.
This study had some limitations, including its small sample size and the fact that it was a single center study. As a retrospective study, enrollment of subjects may have been influenced by selection bias. Some patients with very high levels of peanutsIgE would have been excluded from the OFCs. In this context, anaphylaxis was observed in 31.8% of all reactions, which was about double that observed in other studies.3132 Nevertheless, this is the first Korean study to evaluate the DDP of sIgE antibodies to peanuts and peanut components for predicting the outcome of OFCs. In addition, peanut allergy was confirmed by a challenge test in all subjects who did not have a history of anaphylaxis.
In conclusion, we found that the previously established DDP of peanut-sIgE level may be useful predictor of the outcomes of OFC in Korean children suspected of peanut allergy. Furthermore, Ara h 2 is the most important allergen in Korean children with peanut allergy. The cutoff levels for peanut (10.3 kU/L) and Ara h 2 (4.0 kU/L) established in this study could aid in the diagnosis of peanut allergy in Korean children.
Figures and Tables
Fig. 2
Comparison of level of specific IgE against peanut, Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9 between the peanut-tolerance group and peanut-allergy group. Each box plot indicates an interquartile range (IQR) with median, upper and lower whiskers; upper and lower boundaries (3rd quartile/1st quartile± 1.5 IQR). *P value <0.05. T, peanut-tolerance group; A, peanut-allergy group.

Fig. 3
ROC analysis using specific IgE against peanut and peanut components (Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9) for the diagnosis of peanut allergy. sIgE, specific IgE; PPV, positive predictive value; NPV, negative predictive value.

Table 1
Demographic characteristics of subjects
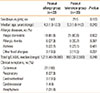
Table 2
Diagnostic Value Of The Prediction Model For The Cutoff Levels Of Specific Ige To Peanut And Ara h 2

Notes
References
1. Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007; 120:491–503.
2. Le TM, Lindner TM, Pasmans SG, Guikers CL, van Hoffen E, Bruijnzeel-Koomen CA, et al. Reported food allergy to peanut, tree nuts and fruit: comparison of clinical manifestations, prescription of medication and impact on daily life. Allergy. 2008; 63:910–916.
3. Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010; 126:324–331. 331.e1–331.e7.
4. Lao-araya M, Trakultivakorn M. Prevalence of food allergy among preschool children in northern Thailand. Pediatr Int. 2012; 54:238–243.
5. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011; 127:594–602.
6. Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010; 125:1322–1326.
7. Sicherer SH, Wood RA. Advances in diagnosing peanut allergy. J Allergy Clin Immunol Pract. 2013; 1:1–13.
8. Kim J, Chang E, Han Y, Ahn K, Lee SI. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatr Allergy Immunol. 2011; 22:715–719.
9. Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in seoul. Allergy Asthma Immunol Res. 2014; 6:131–136.
10. Fleischer DM. The natural history of peanut and tree nut allergy. Curr Allergy Asthma Rep. 2007; 7:175–181.
11. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004; 113:805–819.
12. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010; 125:S116–S125.
13. Kim J, Kim HY, Park MR, Choi J, Shim JY, Kim MJ, et al. Diagnostic decision points of specific IgE concentrations in Korean children with egg and cow's milk allergies. Allergy Asthma Immunol Res. 2015; 7:332–338.
14. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107:891–896.
15. Klemans RJ, Otte D, Knol M, Knol EF, Meijer Y, Gmelig-Meyling FH, et al. The diagnostic value of specific IgE to Ara h 2 to predict peanut allergy in children is comparable to a validated and updated diagnostic prediction model. J Allergy Clin Immunol. 2013; 131:157–163.
16. Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC, et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011; 154:216–226.
17. Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011; 127:684–685.
18. Kim J, Lee JY, Han Y, Ahn K. Significance of Ara h 2 in clinical reactivity and effect of cooking methods on allergenicity. Ann Allergy Asthma Immunol. 2013; 110:34–38.
19. Simons FE, Ardusso LR, Dimov V, Ebisawa M, El-Gamal YM, Lockey RF, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013; 162:193–204.
20. Song TW, Kim KW, Kim WK, Kim JH, Kim HH, Park YM, et al. Guidelines for the oral food challenges in children. Pediatr Allergy Respir Dis. 2012; 22:4–20.
21. Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Standardization of food challenges in patients with immediate reactions to foods--position paper from the European Academy of Allergology and Clinical Immunology. Allergy. 2004; 59:690–697.
22. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009; 123:S365–S383.
23. Wainstein BK, Yee A, Jelley D, Ziegler M, Ziegler JB. Combining skin prick, immediate skin application and specific-IgE testing in the diagnosis of peanut allergy in children. Pediatr Allergy Immunol. 2007; 18:231–239.
24. Roehr CC, Edenharter G, Reimann S, Ehlers I, Worm M, Zuberbier T, et al. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clin Exp Allergy. 2004; 34:1534–1541.
25. Vereda A, van Hage M, Ahlstedt S, Ibañez MD, Cuesta-Herranz J, van Odijk J, et al. Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011; 127:603–607.
26. Flinterman AE, van Hoffen E, den Hartog Jager CF, Koppelman S, Pasmans SG, Hoekstra MO, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy. 2007; 37:1221–1228.
27. Chiang WC, Pons L, Kidon MI, Liew WK, Goh A, Wesley Burks A. Serological and clinical characteristics of children with peanut sensitization in an Asian community. Pediatr Allergy Immunol. 2010; 21:e429–e438.
28. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010; 125:191–197. e1–e13.
29. Dang TD, Tang M, Choo S, Licciardi PV, Koplin JJ, Martin PE, et al. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012; 129:1056–1063.
30. Keet CA, Johnson K, Savage JH, Hamilton RG, Wood RA. Evaluation of Ara h2 IgE thresholds in the diagnosis of peanut allergy in a clinical population. J Allergy Clin Immunol Pract. 2013; 1:101–103.
31. Dyer AA, Rivkina V, Perumal D, Smeltzer BM, Smith BM, Gupta RS. Epidemiology of childhood peanut allergy. Allergy Asthma Proc. 2015; 36:58–64.
32. Ho MH, Lee SL, Wong WH, Ip P, Lau YL. Prevalence of self-reported food allergy in Hong Kong children and teens--a population survey. Asian Pac J Allergy Immunol. 2012; 30:275–284.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download