Abstract
Purpose
MicroRNAs (miRs) were recently recognized to be important for immune cell differentiation and immune regulation. However, whether miRs were involved in allergen-specific immunotherapy (SIT) remains largely unknown. This study sought to examine changes in miR-146a and T regulatory cells in children with persistent allergic rhinitis (AR) after 3 months of subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT).
Methods
Twenty-four HDM-sensitized children with persistent AR were enrolled and treated with SCIT (n=13) or SLIT (n=11) for 3 months. Relative miR-146a and Foxp3 mRNA expression, the TRAF6 protein level, and the ratio of post-treatment to baseline IL-10+CD4+ T cells between the SCIT and SLIT groups were examined in the peripheral blood mononuclear cells (PBMCs) of AR patients using quantitative reverse transcription polymerase chain reaction (qRT-PCR), flow cytometry, and Western blot analysis, respectively. Serum levels of IL-5 and IL-10 were determined using ELISA.
Results
After 3 months of SIT, both the TNSS and INSS scores were significantly decreased compared to the baseline value (P<0.01). The relative expression of miR-146a and Foxp3 mRNA was significantly increased after both SCIT and SLIT (P<0.01). The ratio of post-treatment to baseline IL-10+CD4+ T cells and the serum IL-10 level were significantly increased in both the SCIT and SLIT groups (P<0.01), whereas the TRAF6 protein level and serum IL-5 level were significantly decreased (P<0.01). No significant differences in these biomarkers were observed between the SCIT and SLIT groups.
Allergic rhinitis (AR) and bronchial asthma are allergen-specific IgE-mediated inflammatory reactions which is characterized by excessive eosinophil infiltration, Th2 cytokine responses, and mucus secretion.1 The AR prevalence is increasing worldwide due to changes in the socioeconomic environment, and its burden on sleep and learning is substantial in children.2 Moreover, AR is considered a risk factor for subsequent asthma comorbidity.12 At present, allergen-specific immunotherapy (SIT) has been shown to be highly effective in reducing nasal symptoms and in improving the quality of life. In contrast to symptom-relief pharmacotherapy, SIT is regarded as the only option that may modify the disease and thus potentially prevent the progression from AR to asthma.3
SIT has been administered subcutaneously (subcutaneous immunotherapy [SCIT]) since its introduction a century ago. At present, there is a growing body of evidence regarding the clinical efficacy of SCIT in AR and asthma.4 However, SCIT in children is hampered by the inconvenience of injection and the risk of severe adverse events, including anaphylaxis and, rarely, death. As an alternative, sublingual immunotherapy (SLIT) has been gradually introduced in clinical practice over the last 2 decades with the primary aim of improving safety and convenience.5 The efficacy of SLIT was demonstrated in numerous clinical trials and was confirmed by several meta-analyses.67 Although some aspects still require clarification, SIT has been proposed to exert its anti-inflammatory effect by inducing regulatory T (Treg) cells.8
MicroRNAs (miRs) are non-coding, single-stranded RNAs that are 22 nucleotides in length and regulate gene expression via base-pairing with complementary sequences within mRNA molecules.9 They were recently recognized to be important for immune cell differentiation and immune regulation.10 miRs act by repressing target gene expression via targeting the 3'-untranslated region (UTR) of mRNAs to induce either mRNA degradation, translation inhibition, or both.910 In addition, miR-146a is one of the 2 members of the miR-146 miR family. It has been reported to be involved in a large number of cell activities, such as suppression of cancer growth, inhibition of inflammation, regulation of the immune system, and suppression of allergic inflammation.1112 Furthermore, miR-146a is highly expressed in Treg cells and is induced upon activation of effector T cells and myeloid cells, where miR-146a acts as a negative feedback regulator to limit TRAF6- and IRAK1/2-mediated signalling in inflammatory settings.13 However, whether miR-146a expression is associated with the clinical efficacy of SIT is unknown. To address this issue, we comparably investigated the clinical efficacy of SCIT and SLIT in children with AR after 3-month treatment and analyzed changes in miR-146a and Treg cells (IL-10+CD4+ T cells) before and after treatment.
Twenty-four HDM-sensitized AR children were enrolled in this study. The children were recruited from 4 independent medical centers in Guangzhou, Zhuhai and Foshan in southern China. Their atopic status was evaluated by a skin prick test and/or a CAP test for a panel of common inhalant allergens (i.e., D. pteronyssinus, D. farinae, Blomia tropicalis, Canis familiaris, Felis domesticus, Blattella germanica, A. alternaria, and Artemisia vulgaris). The diagnosis of AR was made according to the international ARIA guidelines.1 Mandatory inclusion criteria were as follows: (1) clinical criteria of moderate to severe persistent AR over the past 2 years; (2) sensitization to house dust mites (HDM), including D. pteronyssinus and/or D. farinae with a positive skin prick test (wheal diameter >6 mm) and/or a CAP-Pharmacia score >class 2 (Phadia, Uppsala, Sweden); (3) age between 4 and 14 years; and (4) FEV1 within the normal limit (>79% of predicted value). The diagnosis of moderate to severe persistent AR was made on the basis of clinical criteria, including nasal rhinorrhea, itching, sneezing, and congestion. Asthma was diagnosed by a physician. Exclusion criteria included (1) children with moderate persistent asthma or anatomic abnormalities of the upper respiratory tract, (2) those undergoing chronic treatment with systemic steroids or with systemic immunological disorders, and (3) those who received intercurrent treatment with β-blockers or oral corticosteroid treatment during the previous 6 months. Treatment with other symptomatic medications (antihistamines, β2-agonists, and/or topical corticosteroids) for AR and/or asthma was permitted during the study period. A total of 20 non-atopic children with obstructive snoring undergoing adenoid surgery were enrolled as healthy controls; these children did not have nasal diseases or a history of asthma. Details of the subjects' characteristics are included in Table 1.
Following the 7-day run-in period, the AR children were re-evaluated for eligibility in an enrolment visit and accepted SCIT and SLIT treatment (n=13 and 11, respectively). This study was approved by the local ethical committee, and the parents of each child provided informed consent.
SCIT was performed with a standardized mite depot-allergen extract (50% D. pteronyssinus and 50% D. farinae) according to the manufacturer's recommendations (Allergopharma Joachim Ganzer KG, Reinbek, Germany). The major allergen contents (D. pteronyssinus and D. farinae) were present in a concentration of 6.4 mg/mL. SLIT was performed using the HDM allergen extract (CHANLLERNGEN, D. farinae Drops) manufactured by Wolwopharma Biotechnology Company (Zhejiang, China). The biologically standardized extracts were labeled with the concentration of total protein and were used in the form of drops (No. 1, 1 µg/mL; No. 2, 10 µg/mL; No. 3, 100 µg/mL; and No. 4, 333 µg/mL). The SCIT and SLIT protocols were performed in strict accordance with the manufacturers' in structions as described elsewhere.1415
All the patients recorded their daily nasal symptom scores throughout the 3-month SIT study. The questionnaires covered symptoms and medication use (i.e., antihistamines and corticosteroids; only used during the first month). The severity of the individual nasal symptom score (INSS), including nasal rhinorrhea, sneezing, itching, and congestion, was assessed on a scale of 0 to 3 (0=no symptoms, 1=mild symptoms, 2=moderate symptoms, and 3=severe symptoms). The total nasal symptom score (TNSS) was defined as the sum of the scores of nasal rhinorrhea, sneezing, itching, and congestion.
The nasal mucosa was sampled from the inferior turbinate of the AR children and healthy controls under local anaesthesia. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples collected from the AR children before and after the 3-month SIT by Ficoll-Hypaque density gradient centrifugation (TBD, Tianjin, China). As described elsewhere,14 total RNA was extracted from the nasal tissues using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Reverse transcription (RT) was performed, and cDNA was synthesized from 2 µg of total RNA using an oligo(dT)18 primer and M-MLV reverse transcriptase (TAKARA, Syuzou, Shiga, Japan) for quantitative PCR. Then, qRT-PCR was performed using the ABI PRISM 7500 Detection System (Applied Biosystems, Foster City, CA, USA) and SYBR Premix Taq™ (TAKARA).14 The primer sequences were as follows: Foxp3 forward, 5'- ACA GTC TCT GGA GCA GCA GC -3'; Foxp3 reverse, 5'- CCA CAG ATG AAG CCT TGG TC -3'; β-actin forward, 5'-AAG ATG ACC CAG ATC ATG TTT GAG ACC-3'; β-actin reverse: 5'- AGC CAG GTC CAG ACG CAG GAT -3'. miR-146a forward, 5'- CAG GAT GAG AAC TGA AT -3'; miR-146a reverse, 5'- GTG CAG GGT CCG AGG T -3'; U6 forward, 5'- CTC GCT TCG GCA GCA CAT AT -3'; and U6 reverse: 5'- TTG CGT GTC ATC CTT GCG -3'. The mean value of the replicates for each sample was calculated and expressed as the cycle threshold (Ct). The mRNA expression level was calculated as the difference (ΔCt) between the Ct value of the target gene and the Ct value of the inner control. The fold change in the target gene mRNA levels was calculated as 2-ΔΔCt.
Venous blood samples were collected into Vacuette tubes and were centrifuged at 3,000 g at 4℃ for 15 minutes. Serum samples were stored at -80℃ for ELISA analysis. Commercially available ELISA kits were used to measure the levels of serum IL-5 and IL-10 (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocols. The detection limit of the assays for IL-5 and IL-10 was 3.9 pg/mL each.
For in vitro nasal epithelial cell (NEC) isolation and culture, the nasal mucosa was sampled from 3 healthy controls via enzymatic digestion as described elsewhere.16 The collected NECs were cultured as submersion cultures in BEGM medium (Lonza, Walkersville, MD, USA) until passaging. At 80%-90% confluency, the cells were stimulated with recombinant human IL-5, IL-13, IL-33, and thymic stromal lymphopoietin (TSLP) (all at concentrations of 50 ng/mL; R&D Systems) for 12 hours. Then, the cell pellets were collected for qRT-PCR.
Western blotting was performed as reported elsewhere.16 Briefly, total proteins were extracted from the isolated PBMCs in 100 µL of RIPA lysis buffer. The protein concentration in the supernatants was determined using the BCA method. Samples containing 20 µg of protein were boiled and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 8% Tris-glycine gels. The separated proteins were electrophoretically transferred to a polyvinylidene fluoride membrane. The membrane was incubated in 5% fat-free skim milk in Tris-buffered solution (TBS) containing 0.05% Tween-20 (1 hour at room temperature) and then incubated with mouse anti-human TNF-α receptor-associated factor (TRAF6) (Abcam, Cambridge, MA, USA) and β-actin monoclonal antibodies (Santa Cruz) diluted 1:2,000 overnight at 4℃. The membrane was washed and incubated in goat anti-mouse IRDye 800 and goat anti-rabbit Alexa Fluor 680 antibodies (Invitrogen) for one hour. Then, the membrane was washed 3 times with TBS-Tween and visualized using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). The membrane was scanned at 700 and 800 nm, and the results were analyzed using Odyssey® software v1.2.
Flow cytometric analysis was performed as described elsewhere.17 Briefly, PBMCs from the AR children before and after SIT treatment and the healthy controls were isolated by Ficoll-Hypaque density gradient centrifugation. For CD4 staining, the cells were incubated with the CD4 mAb (eBioscience, San Diego, CA, USA) at 4℃ in the dark for 30 minutes. Following fixation and permeabilization with Permeabilization/Fixation buffer (BD Biosciences), the cells were stained with conjugated mAbs for IL-10 (eBioscience) according to the protocol of the Permeabilization/Fixation Kit. The stained cells were washed twice prior to analysis using the FACS Aria II cytometer (BD Biosciences).
Data are expressed as the medians and interquartile ranges except where otherwise indicated. These data were analyzed via the Kruskal-Wallis H and nonparametric Mann-Whitney U tests. For the in vitro experiments, the data were analyzed via 1-way ANOVA and Student's t test. The correlation between different biomarkers in AR children was assessed using Pearson's correlation test. A P value of less than 0.05 was considered significant.
In this study, a total of 24 children with HDM-sensitized AR were enrolled after a 7-day run-in period. The children were recruited from 4 independent medical centers in Guangzhou, Zhuhai, and Foshan in southern China. The overall demographic and clinical characteristics are presented in Table 1. The majority of subjects were Han nationality (95.8%), and the mean age and duration were 6.5 and 2.9 years, respectively. The coexisting rate of asthma was low (8.3%), and the monosensitized subjects shared a population of 91.7%.
After 3 months of SCIT or SLIT treatment (n=13 and 11, respectively), all INSS (nasal rhinorrhea, itching, sneezing, and congestion), and TNSS significantly decreased compared to the baseline values (Table 2). However, no significant difference was observed in INSS or TNSS between the SCIT and SLIT groups after 3 months of treatment.
The expression of miR-146a and the Foxp3 mRNA was detected in the PBMCs of all the AR children (n=24) and healthy controls (n=20) using qRT-PCR. As shown in Fig. 1, the levels of miR-146a and Foxp3 mRNA were significantly decreased in the PBMCs of the AR children compared to the healthy controls (P<0.01). Moreover, the level of miR-146a was positively associated with the Foxp3 mRNA level (r=0.69, P<0.01), but was negatively associated with the disease severity (TNSS) (r=-0.60, P<0.05). Consistently, when the level of miR-146a was examined in the nasal mucosa of the AR patients (n=9) and healthy controls (n=9) using qRT-PCR, we found that miR-146a was detected in all the AR subjects and that the level of miR-146a was significantly decreased in the nasal mucosa of the AR children compared to the healthy controls (P<0.01) (Fig. 2A). Next, we stimulated cultured healthy NECs with IL-5, IL-13, IL-33, and TSLP (50 ng/mL for 12 hours) to evaluate the potential regulation of miR-146a expression by Th2-related cytokines in NECs. Consequently, we found that these cytokines significantly inhibited miR-146a expression in the NECs (IL-5, 30.7%; IL-13, 44.3%; IL-33, 50.3%; and TSLP, 25.4%) (P<0.05).
Next, we examined changes in miR-146a, Foxp3 and other biomarkers in the 2 groups of AR children after 3 months of SCIT or SLIT treatment (SCIT, n=8 and SLIT, n=7). Using qRT-PCR analysis, we found that the levels of miR-146a and Foxp3 mRNA in the PBMCs from the AR children were significantly increased compared to their baseline values (P<0.01); no significant difference was observed between the SCIT and SLIT groups (Fig. 3). Next, we examined the expression of the TRAF6 protein in PBMCs from the AR children before and after SIT treatment and in the PBMCs from the healthy controls using Western blot analysis (SCIT, n=5, SLIT, n=5, and healthy control, n=5). As shown in Fig. 4, the TRAF6 protein levels in the PBMCs of the AR children were significantly increased compared to the healthy controls (P<0.01). After 3 months of SIT treatment, the TRAF6 protein levels in the PBMCs of the AR children in both the SCIT and SLIT groups were significantly decreased compared to their baseline values (P<0.01). Similarly, as shown in Fig. 5, the IL-5 level was significantly decreased, and IL-10 level was significantly increased in both the SCIT and SLIT groups after 3 months of treatment compared to their baseline values (P<0.01). No significant differences in the serum IL-5 and IL-10 levels were observed between the SCIT and SLIT groups. Additionally, we examined the ratio of post-treatment to baseline IL-10+CD4+ T cells in the PBMCs of the AR children after 3 months of SIT treatment between the 2 groups. As shown in Fig. 6, the ratio of post-treatment to baseline IL-10+CD4+ T cells between the SCIT and SLIT groups were significantly increased after 3 months of treatment compared to their baseline values (P<0.01). There was no significant difference in the ratio of post-treatment to baseline IL-10+CD4+ T cells between the SCIT and SLIT groups.
The clinical implication of miR-146a in AR patients is unknown. In this study, we demonstrated a significant decrease in miR-146a expression in AR children that was positively associated with disease severity. Moreover, we found that miR-146a was significantly up-regulated in AR children after 3 months of both SCIT and SLIT treatments. The results of this study support an important therapeutic role for miR-146a in the induction of immune tolerance in AR patients, which expands our understanding of the pathophysiology and treatment of AR.
The current treatment of AR includes the avoidance of allergens, pharmacotherapy, and SIT.2 In contrast to symptom suppression by pharmacotherapy, SIT aims to alter the immune system and may represent a cure for AR. SCIT is a well-established treatment for allergic disease and is accompanied by reductions in IgE levels and the inflammatory response. A meta-analysis showed that SCIT is effective in AR patients and results in a long-lasting reduction in symptoms and drug requirements; moreover, it seemed to prevent new sensitization and asthma.6 However, the SCIT regimens require multiple injections and frequent visits to the physician's office and are limited by safety concerns due to the risks of IgE-mediated side effects. Moreover, it has also been suggested that injections can be traumatic for young children, leading to increased difficulty in cooperation with an immunotherapy program. SLIT is currently recommended as an alternative suitable option for AR patients. We previously demonstrated that both TNSS and INSS scores were significantly decreased compared to their baseline values in AR children after 6 months of SLIT.14 In this study, we compared the clinical efficacy of SCIT and SLIT in AR children and found that SCIT and SLIT comparably improved nasal symptoms after a 3-month treatment. These findings were consistent with those of the previous study showing that the clinical efficacy of SLIT (symptoms and medication use) was equivalent to SCIT in a double-dummy study conducted in grass pollen allergic patients.18 This suggests that SLIT may be particularly recommendable due to its comparable clinical efficacy, improved ease of administration, painlessness and safety, with little risk of systemic adverse effects in children with AR.
The immunological basis of allergic disease includes sensitization, the development of memory T- and B-cell responses, and IgE-mediated tissue inflammation and injury. SIT is a successful therapy for allergic disease and offers the advantages of specificity and long-lasting efficacy.5 Reported immunological mechanisms of effective SIT include the down-regulation of adverse Th2 immune responses to allergens, immune deviation, blocking antibody production, and Treg cell induction.19 The change in specific IgE is not an immunological indicator of successful SIT because many studies on SIT have reported that there are no changes in specific IgE after treatment. However, induction of Treg cells, which is initiated by the cytokines IL-10 and transforming growth factor-β (TGF-β), has been proposed to be an essential step in allergen-specific SIT.20 In previous studies, we and other investigators demonstrated that SLIT also significantly increases the immune-suppressive cytokines IL-10 and TGF-β in AR patients.142122 However, the immunological changes after SIT treatment are not completely understood.
Recent studies have revealed the critical roles of specific miRNAs in the regulation of key pathogenic mechanisms of allergic inflammation, including polarization of adaptive immune responses and activation of T cells, regulation of eosinophil development, and modulation of epithelial responses.10 In particular, miR-146a is widely expressed and is involved in the differentiation and activation of both the innate and adaptive immune systems. It was initially reported to be up-regulated in macrophages in response to TLR-mediated NF-κB signaling. A previous study suggested that miR-146a was an important player in the NF-κB signaling pathway in innate immunity because it down-regulated the NF-κB signaling transducers TRAF6 and calmed NF-κB signals.23 More recently, miR-146a was implicated in T cell regulation in several inflammatory disorders.2425 For example, Tsitsiou et al.26 showed that miR-146a is reduced in CD8+ and CD4+ T cells in patients with severe asthma. Lu et al.11 reported that miR-146a controls the suppressor function of Treg cells by targeting signal transducer and activator of transcription 1. These studies suggested that miR-146a may play a role in SIT of allergic diseases by modulating Treg cells.
Several studies screened the expression of miRs in AR patients. For example, Yu et al.2728 reported a decreased expression of miR-224, miR-143, and miR-187 in AR patients by means of microarray analysis of differentially expressed miRs and also demonstrated that miR-143 modulates mucus production by targeting the IL-13 receptor α subunit. Suojalehto et al.29 also reported a decreased level of let-7e in AR patients compared to non-allergic subjects. However, the role of miR-146a in AR has not been well documented. To address this issue, we first examined miR-146a expression in the PBMCs and nasal mucosa of AR patients. Consequently, we found significantly decreased miR-146a expression in the PBMCs and nasal mucosa and the ratio of post-treatment to baseline IL-10+CD4+ Treg cells in AR patients compared to the healthy controls. Interestingly, we found that miR-146a expression in NECs was significantly inhibited by Th2-related cytokines, such as IL-5, IL-33, IL-33, and TSLP. Moreover, altered miR-146a expression in the PBMCs of AR children was associated with Foxp3 expression and disease severity. These findings suggest miR-146a may be involved in the insufficiency of Treg cells for AR pathogenesis. Next, we examined miR-146a expression in both the SCIT and SLIT groups after 3 months of treatment to investigate the critical association of miR-146a with Treg cells in AR patients during SIT treatment,. Consistent with the improved nasal symptoms, we found comparable changes in miR-146a, Foxp3, TRAF6, and Treg cells in the PBMCs, and IL-5 and IL-10 levels in the serum of AR children. To the best of our knowledge, this is the first report concerning changes in miR-146a in AR children after SIT treatment. Our findings suggested that miR-146a can be a promising biomarker for the management of AR by inducing immune tolerance.30 We acknowledge that this study contains several limitations. This is a short-term observatory case-control study with a small sample size, and no placebo group of AR patients was enrolled. Thus, bias cannot be completely avoided. Further long-term studies with a larger sample size and a placebo group are required to address the importance of miR-146a in the SIT treatment of AR.
In conclusion, we showed that miR-146a expression was significantly decreased in AR patients and was in turn significantly associated with Foxp3 expression and the nasal symptom score. Moreover, we found that miR-146a expression and the nasal symptom score were comparably modulated after 3-month SCIT and SLIT treatments. Our results suggest that miR-146a may be considered a promising biomarker for the pathogenesis and management of AR patients.
Figures and Tables
Fig. 1
The expression of miR-146a and Foxp3 mRNA in the PBMCs of AR children (n=24) and healthy controls (n=20). The levels of miR-146a (A) and Foxp3 mRNA (B) were significantly decreased in the PBMCs of AR children compared to the healthy controls. The level of miR-146a was positively associated with the Foxp3 mRNA level (C), but was negatively associated with the disease severity (TNSS) (D). *P<0.01.

Fig. 2
The expression and regulation of miR-146a in the nasal mucosa of AR children (n=9) and healthy controls (n=9). (A) The level of miR-146a was significantly decreased in the nasal mucosa of AR children compared to the healthy controls. (B) The level of miR-146a in cultured healthy nasal epithelial cells was significantly inhibited by Th2-related cytokines (IL-5, IL-13, IL-33, and TSLP; 50 ng/mL for 12 hours). The data are expressed as the means (SEM) of the 3 independent experiments. *P<0.01; **P<0.05.

Fig. 3
The expression of miR-146a and Foxp3 mRNA in the PBMCs of AR children after a 3-month SIT treatment. After 3-months of SCIT and SLIT treatments, the miR-146a (A) and Foxp3 mRNA (B) levels were significantly increased compared to their baseline values. AR1, untreated AR children; AR2, AR children with a 3-month SCIT treatment; AR3, AR children with a 3-month SLIT treatment. *P<0.01.
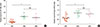
Fig. 4
The expression of TRAF6 in the PBMCs of the AR children after a 3-month SIT treatment. (A) Representative Western blot analysis of TRAF6 in the PBMCs of AR children and healthy controls was shown. (B) The level of the TRAF6 protein in the PBMCs of the AR patients was significantly increased compared to the healthy controls. (C) Representative Western blot analysis of TRAF6 in the PBMCs of the AR children before and after SIT treatment. (D) After 3 months of SCIT or SLIT treatment, the level of the TRAF6 protein in both the SCIT and SLIT groups was significantly decreased compared to their baseline values. No significant difference in the TRAF6 protein level was observed between the SCIT and SLIT subgroups. AR1, untreated AR children; AR2, AR children with a 3-month SCIT treatment; AR3, AR children with a 3-month SLIT treatment. *P<0.01.

Fig. 5
Serum IL-5 and IL-10 levels in AR children after a 3-month SIT treatment. After a 3-month SCIT or SLIT treatment, a significant decrease in the IL-5 level and an increase in the IL-10 level were observed in both the SCIT and SLIT groups compared to their baseline values. No significant difference in serum IL-5 and IL-10 levels was observed between the SCIT and SLIT groups. AR1, untreated AR children; AR2, AR children with a 3-month SCIT treatment; AR3, AR children with a 3-month SLIT treatment. *P<0.01.
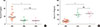
Fig. 6
The ratio of IL-10+CD4+ T cells in the PBMCs of AR children before and after a 3-month SIT treatment. (A) Representative flow cytometric analysis of IL-10+CD4+ T cells in the PBMCs of the AR children was shown. (B) After a 3-month SCIT or SLIT treatment, the ratios of IL-10+CD4+ T cells in both the SCIT and SLIT subgroups were significantly increased compared to their baseline values. No significant difference in the ratio of IL-10+CD4+ T cells was observed between the SCIT and SLIT subgroups. AR1, untreated AR children; AR2, AR children with a 3-month SCIT treatment; AR3, AR children with a 3-month SLIT treatment. *P<0.01.

Table 1
The demographics and clinical characteristics of the study subjects

Table 2
Change in the nasal symptom score after a 3-month SIT treatment

ACKNOWLEDGMENTS
This study was supported by the National Natural Science Grants of China (No. 81271054, 81271056, 81470673) and grants from the Ministry of Hygiene (no. 201202005, 2014BAI07B04). The authors have declared that they have no conflict of interest.
References
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.
2. Zhang Y, Zhang L. Prevalence of allergic rhinitis in china. Allergy Asthma Immunol Res. 2014; 6:105–113.
3. Cibella F, Ferrante G, Cuttitta G, Bucchieri S, Melis MR, La Grutta S, et al. The burden of rhinitis and rhinoconjunctivitis in adolescents. Allergy Asthma Immunol Res. 2015; 7:44–50.
4. Braido F, Arcadipane F, Marugo F, Hayashi M, Pawankar R. Allergic rhinitis: current options and future perspectives. Curr Opin Allergy Clin Immunol. 2014; 14:168–176.
5. Wang C, Zhang L. Specific immunotherapy for allergic rhinitis in children. Curr Opin Otolaryngol Head Neck Surg. 2014; 22:487–494.
6. Di Bona D, Plaia A, Scafidi V, Leto-Barone MS, Di Lorenzo G. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2010; 126:558–566.
7. Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol. 2012; 130:1097.e2–1107.e2.
8. Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011; 127:18–27.
9. Rebane A, Akdis CA. MicroRNAs: essential players in the regulation of inflammation. J Allergy Clin Immunol. 2013; 132:15–26.
10. Lu TX, Rothenberg ME. Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J Allergy Clin Immunol. 2013; 132:3–13.
11. Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010; 142:914–929.
12. Rebane A, Runnel T, Aab A, Maslovskaja J, Rückert B, Zimmermann M, et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol. 2014; 134:836.e11–847.e11.
13. Park H, Huang X, Lu C, Cairo MS, Zhou X. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J Biol Chem. 2015; 290:2831–2841.
14. Lin Z, Zhou L, Luo X, Xia W, Chen D, Xu R, et al. Suppression of TIM-1 predicates clinical efficacy of sublingual immunotherapy for allergic rhinitis in children. Int J Pediatr Otorhinolaryngol. 2013; 77:1345–1349.
15. Peng H, Li CW, Lin ZB, Li TY. Long-term efficacy of specific immunotherapy on house dust mite-induced allergic rhinitis in China. Otolaryngol Head Neck Surg. 2013; 149:40–46.
16. Luo Q, Zhang J, Wang H, Chen F, Luo X, Miao B, et al. Expression and regulation of transcription factor foxA2 in chronic rhinosinusitis with and without nasal polyps. Allergy Asthma Immunol Res. 2015; 7:458–466.
17. Xiao L, Wei Y, Zhang YN, Luo X, Yang BY, Yu SF, et al. Increased IL-21 expression in chronic rhinosinusitis with nasalpolyps. Clin Exp Allergy. 2015; 45:404–413.
18. Quirino T, Iemoli E, Siciliani E, Parmiani S, Milazzo F. Sublingual versus injective immunotherapy in grass pollen allergic patients: a double blind (double dummy) study. Clin Exp Allergy. 1996; 26:1253–1261.
19. Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012; 18:736–749.
20. Palomares O, Martín-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-β. Genes Immun. 2014; 15:511–520.
21. Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007; 120:707–713.
22. O'Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009; 180:936–947.
23. Li S, Yue Y, Xu W, Xiong S. MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS One. 2013; 8:e81438.
24. Dalbeth N, Pool B, Shaw OM, Harper JL, Tan P, Franklin C, et al. Role of miR-146a in regulation of the acute inflammatory response to monosodium urate crystals. Ann Rheum Dis. 2015; 74:786–790.
25. Baldeón RL, Weigelt K, de Wit H, Ozcan B, van Oudenaren A, Sempértegui F, et al. Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PLoS One. 2014; 9:e115209.
26. Tsitsiou E, Williams AE, Moschos SA, Patel K, Rossios C, Jiang X, et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J Allergy Clin Immunol. 2012; 129:95–103.
27. Shaoqing Y, Ruxin Z, Guojun L, Zhiqiang Y, Hua H, Shudong Y, et al. Microarray analysis of differentially expressed microRNAs in allergic rhinitis. Am J Rhinol Allergy. 2011; 25:e242–e246.
28. Teng Y, Zhang R, Liu C, Zhou L, Wang H, Zhuang W, et al. miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1. Biochem Biophys Res Commun. 2015; 457:58–64.
29. Suojalehto H, Toskala E, Kilpeläinen M, Majuri ML, Mitts C, Lindström I, et al. MicroRNA profiles in nasal mucosa of patients with allergic and nonallergic rhinitis and asthma. Int Forum Allergy Rhinol. 2013; 3:612–620.
30. Rhee CS. Current specific immunotherapy for allergic rhinitis: perspectives from otorhinolaryngologists. Allergy Asthma Immunol Res. 2014; 6:273–275.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download