Abstract
Purpose
In the celery-mugwort-birch-spice syndrome, a significant proportion of IgE is directed against high molecular weight (HMW) glycoproteins, including the celery allergen Api g 5. BIP3, a monoclonal antibody originally raised against birch pollen, recognizes HMW allergens in birch and mugwort pollens, celery, and Apiaceae spices. Our aim was to generate mimotopes using BIP3 for immunization against the HMW allergens relevant in the celery-mugwort-birch-spice cross reactivity syndrome.
Methods
Mimotopes were selected from a random-peptide display library by BIP3 and applied in IgE inhibition assays. The 3 phage clones with the highest inhibitory capacity were chosen for immunization of BALB/c mice. Mouse immune sera were tested for IgG binding to blotted birch pollen extract and used for inhibiting patients' IgE binding. Furthermore, sera were tested for binding to Api g 5, to horseradish peroxidase (HRP) as a second glycoprotein, or to non-glycosylated control allergen Phl p 5 in ELISA, and the specific Api g 5-specific IgG titers were determined.
Results
Three rounds of biopanning resulted in phage clones exhibiting 7 different sequences including 1 dominant, 1-6-cyclo-CHKLRCDKAIA. Three phage clones had the capacity to inhibit human IgE binding and induced IgG to the HMW antigen when used for immunizing BALB/c mice. The induced BIP3-mimotope IgG reached titers of 1:500 specifically to Api g 5, but hardly reacted to glycoprotein HRP, revealing a minor role of carbohydrates in their epitope.
Conclusions
The mimotopes characterized in this study mimic the epitope of BIP3 relevant for Api g 5, one of the cross-reactive HMW allergens relevant in the celery-mugwort-birch-spice syndrome. BIP3 mimotopes may be used in the future for hyposensitization in this clinical syndrome by virtue of good and specific immunogenicity.
Allergy to plant-derived food is often associated with a primary sensitization to pollen allergens. A well-known and clinically important phenomenon is the "celery-mugwort-birch-spice syndrome", which occurs in adults12345 as well as in children.6 The additional relationship between mugwort and ragweed may possibly even extent this cross-reactivity syndrome.7 Sensitization against celery tuber represents one of the clinically most important causes of food allergies, especially in European countries such as Switzerland, Germany, and France.8910 Derived from the same botanical Apiaceae family, the cross-reactive spices anise, fennel, cumin, and coriander may complicate the diagnosis.1 In the celery-mugwort-birch-spice syndrome, IgE cross-reactivity is associated with several classes of allergens: (1) Bet v 1, Api g 1, and other PR-10 allergens, (2) profilins such as Bet v 2 or Api g 4,11 (3) non-specific lipid transfer proteins,1213 and (4) high molecular weight (HMW) allergens (32-70 kDa), of which only Api g 5 from celery and its homologues from grass pollen and fennel have been identified so far.1416 Sensitization to Api g 5 was detected in up to 42% of patients with celery allergy, and there was an excellent correlation between sensitization to Api g 5 and to cross-reactive N-glycan epitopes.1517 Monoclonal antibody, BIP3 was originally raised against birch pollen HMW allergens between 32 and 63 kDa.5 It also reacts to mugwort pollen and celery antigens at 55-58 kDa, which were previously characterized by N-terminal sequencing.1718 The resulting glycoproteins turned out to be size variants of Api g 5.19
In the present study, we aimed to generate peptide mimotopes using this cross-reactive antibody BIP3, in analogy to previous studies.20 Therefore, we screened a peptide phage display library with BIP3 and immunized BALB/c mice with the phagedisplayed BIP3-specific mimotopes. The immune sera were used to reveal reactivity towards glycoprotein Api g 5. Furthermore, we addressed the possible participation of carbohydrate moieties, i.e. N-glycans in the BIP3 epitope by testing for cross-reactivity to horseradish peroxidase (HRP) as an ideal model glycoprotein representing multiple carbohydrate species21: Whereas bromelain from pineapple stem has only one xylose- and fucose-containing N-linked glycan (MUXF), HRP has 6 xylose and fucose containing glycans (MMXF). This is important as Api g 5 has both types of fucosylated and xylosylated complex N-glycans, namely, MMXF and MUXF.17 It should be noted that MUXF from bromelain lacks a third mannose residue which is found in many plant glycoproteins like on HRP and Api g 5. This might influence, to some extent, antibody binding as bromelain has been found less sensitive for detection of CCD-positive sera than HRP and Api g 5. Our hypothesis was that mimotopes could be used for improved diagnosis and immunotherapy of the celery-mugwort-birch-spice syndrome.
The production of mAbs BIP3 and BIP1 has been described by Jarolim et al.5 In that study, birch pollen extract was used for immunizing BALB/c mice. Among the monoclonals raised, some (e.g. BIP1 and BIP4) were directed against the major allergen Bet v 1, whereas only BIP3 was directed against HMW allergens. Sera from birch pollen allergic patients cross-reacting to celery were sampled in the Vienna outpatient clinic Allergy-Care®. The usage of serum remnants from routine diagnosis was permitted in written form by the patients, and the study was approved by the ethics committee of the Medical University of Vienna, according to the Helsinki guidelines.
Birch and mugwort pollen were purchased from Allergon AB (Engelholm, Sweden) and were extracted (10% weight/volume) using 10 mM/L potassium phosphate buffer (pH 7.0) overnight under continuous agitation at 4℃. After 1 hour centrifugation at 4℃ (40,000 g), supernatants were dialyzed (cutoff 6,000-8,000 Da) against 10 mM/L K2HPO4 and 3 mM/L sodium azide for two days. Thereafter, dialysates were lyophilized and stored at -20℃. Apiaceae spices (anise; coriander; cumin; fennel) and celery tubers were purchased at a local store, ground in liquid nitrogen, and extracted as described above. Protein content of extracts was determined by Bradford assay.22 Api g 5 was purified as described by Bublin et al.17 HRP was purchased from Fluka, and the plasmid for the production of rPhl p 5 was kindly provided by Prof. A. Petersen, Research Center, Borstel, Germany.
Protein extracts were loaded onto gels in the following concentrations: coriander 50 µg/lane, cumin 50 µg/lane, fennel 50 µg/lane, anise 50 µg/lane, mugwort pollen 50 µg/lane, celery 25 µg/lane, birch pollen 25 µg/lane, Api g 52 µg/lane, and HRP 4 µg/lane. They were separated by preparative 8% or 12% SDS-PAGE under reducing conditions. Separated proteins were transferred to activate PVDF membrane (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Blot strips were saturated with blocking buffer 5% dry milk in Tris buffered saline (TBS) with 0.05% Tween-20 (TBST).
The mAb BIP3 and the Bet v 1-specific mAb BIP1 were diluted to 1 µg/mL in blocking buffer; the sera of mimotope-immunized mice (see below) were diluted 1:100 and incubated with blot strips at 4℃ overnight. After washing with TBST, bound mouse mAbs were detected by125 I-labelled sheep anti-mouse Ig (Amersham Life Science, Little Chalfont, UK) and blots autoradiographed on Kodak Biomax MS films at -70℃.
For human IgE detection, after saturation of blot strips, individual human sera diluted 1:5 in blocking buffer were incubated overnight. After washing with TBST, bound IgE was detected by a peroxidase-labeled anti-human IgE (diluted 1:3,000) (KPL, Gaithersburg, MD, USA). After washing, the reaction was developed with Clarity ECL Western substrate (BioRad, Contra Costa County, CA, USA), and data were acquired by using ChemiDoc Touch Imaging System (BioRad, CA, USA). Alternatively for competition assays, blots were first pre-incubated with a pool of 5 mouse mimotope sera and were incubated with a patient's serum 1:5 after stringent washing with TBST. IgE was detected as described.
Deglycosylation of blotted proteins was performed by 3 hours of incubation of nitrocellulose membranes in 0.01 M NaIO4 in acetate buffer (50 mM pH 4.5) at 4℃ under continuous shaking while protected from light. The reaction was terminated by washing in aqua bidest.
BIP3 specific phage clones were selected from a random peptide phage library expressing circular (pVIII9aa.Cys) nonapeptides fused to pVIII of the filamentous bacteriophage fd.23 Libraries were kindly provided by IRBM (Istituto di Recherche di Biologia Molecolare P. Angeletti SPA, Rome, Italy).
Three rounds of biopanning were performed using 1 µg of monoclonal antibody BIP3 in 0.1 M NaHCO3, pH 8.5 for coating on ELISA plates (Nunc, Roskilde, Denmark) overnight at 4℃ in a humid chamber. After blocking using PBS containing 3% BSA for 1 hour at 37℃, 1011 plaque-forming units (pfu) of phage library or eluent from the previous round of panning were added to each well and incubated at 37℃ for 2 hours. Unbound phages were removed by washing with TBS/0.5% Tween 20. BIP3-binding eluted using 0.1 M HCl pH 2.2 containing 1 mg/mL, and pH was adjusted to 7.0 with 1 M Tris-HCl, pH 8. These phage clones were used for further rounds of biopanning. The enrichment of BIP3-specific phage particles was confirmed by colony screening.
After each biopanning round, eluted phages were propagated in E. coli XL-1 Blue co-infected with helper phage VCSM13 (Stratagene, La Jolla, CA, USA) in liquid culture. An aliquot of phages was further cultivated on LB/carbenicillin plates, and the total number of eluted phages was calculated by counting colonies. Sixty colonies/biopanning round were randomly picked, transferred to new LB/carbenicillin plates, and grown overnight at 37℃. The colonies were then incubated for 1 day covered with an isopropyl-β-D-1-thiogalactopyranoside (IPTG)-treated nitrocellulose. After fixing the colonies to the nitrocellulose by incubation in a chloroform atmosphere and 30-minute blocking using 50 mM Tris, 150 mM NaCl, 5 mM MgCl2, and 3% (w/v) BSA, pH 8.0, cells were lysed using blocking buffer additionally containing 400 mg/mL lysozyme (Sigma, St. Louis, MO, USA) and 20 U/mL DNAse (Boehringer-Mannheim, Mannheim, Germany) for 1 hour at room temperature. Then the filters were saturated with PBS containing 0.5% BSA and 0.5% Tween 20 for 1 hour at room temperature. Afterwards, the membrane was screened for BIP3-specific phage clones using antibody BIP3 in comparison to BIP1 as isotype control antibody and detection performed as described in section "SDS-PAGE and Western blot." Positive clones were re-amplified in overnight cultures and stored in 20% glycerol at -70℃.
BIP3-specific clones were lysed and DNA was isolated by precipitation with polyethylene glycol 8000 (Amresco, Dallas, TX, USA). DNA sequencing was performed by the Sanger dideoxy method using a Thermo Sequenase Cycle Sequencing Kit (Amersham Life Science) with fluorescence-labeled primer 5'-GCT TTA CAC TTT ATG CTT-3' (Eurofins, Ebersberg, Germany) using a LI-COR DNA sequencer 4000L (LI-COR Inc., Lincoln, NE, USA). Phylogenetic alignments were constructed using Vector NTI Advance 11.5.0 (Invitrogen, Carlsbad, CA, USA).
Birch pollen allergic patients' sera were diluted 1:5 in blocking buffer or in blocking buffer containing 107 cfu/mL of single BIP3-mimotope clones from the third biopanning round. As negative controls, the original library, helper phage (VSCM13), or a phage clone of irrelevant specificity (CRQTRTRTMPGCG) (107 cfu each), were used. Diluted serum samples were then incubated on blotted birch pollen extract at 4℃ overnight and IgE detected as described above.
BALB/c mice (female; 3-5 weeks) were purchased from Charles River Labs, Sulzheim, Germany and were treated according to the European community rules of animal care. Immunizations were performed with the permission of the Austrian Ministry of Science (BMWF-66.009/0170-II/10b/2009), and institutional and national guidelines for the care and use of laboratory animals were followed.
Mice were immunized intraperitoneally (i.p.) with either of the following mimotope phage clones without adjuvants: 1-12-cyclo-CKASSCDTGHC; 1-12-cylo-CFFAWRSLPNC, and 1-6-cyclo-CHKLRCDKAIA on days 0, 14, and 28 using 1013 phage particles/100 µL 0.9% NaCl/PBS. Phage clone CHKLR CDKAIA was used for immunization of additional BALB/c mice (n=6) according to the same treatment scheme. Blood was taken on days 0 (pre-immune serum), 21, and 38.
Microtitre plates (Maxisorp, Nunc, Roskilde, Denmark) were coated overnight at 4℃ with 1 µg/mL of Api g 5, HRP, or for control, rPhl p 5 in 50 mM NaHCO3, pH 9.6 overnight at 4℃. Plates were washed with Tris-buffered saline containing 0.05% Tween 20 (TBST) and blocked for 2 hours at room temperature with TBST/1% bovine serum albumin (BSA). Sera from the immunisations with phage CHKLRCDKAIA were pooled to equal parts, diluted 1:100 in TBST/0.1% BSA, and incubated overnight at 4℃. Plates were washed, and alkaline phosphatase labeled anti-mouse IgG/M in TBST/0.1% BSA was added for 2 hours at room temperature. Reaction was developed with para-nitrophenylphosphate in 0.2 M/L Tris buffer. Optical density was measured at 405 nm using microplate reader Tecan infinite M200-Pro (Tecan Group Ltd., Männedorf, Switzerland).
For mouse IgG titer determination, plates were coated with 10 µg/ml Api g 5 and incubated with serial dilutions 1:10-1:10,00 of anti-BIP3 mimotope CHKLRCDKAIA mouse serum pool (5 sera pooled at equal parts) or pre-immune serum pool of the same mice. Bound IgG was detected with a 1:5,000 dilution of goat anti mouse IgG-Fc Fragment-HRP (Bethyl Laboratories Inc., Montgomery, TX, USA), ABTS was used for color development. Optical density was measured at 405 nm.
The monoclonal anti-birch pollen antibody BIP3 reacted to HMW allergens at 35-77 kDa in birch pollen; its binding was almost, but not completely, abolished when N-glycans were chemically removed (Fig. 1A).
BIP3 also exhibited binding to HMW allergens in birch, celery, and mugwort pollen extracts (Fig. 1B), which is indicative of the cross-reactivity spectrum typically seen in sera of patients with the celery-mugwort-birch-spice syndrome. For comparison, lane 1 in Figure 1B shows varying binding intensity of the anti-Bet v 1 antibody BIP15 to the same extracts.
Supplemental Figure 1 shows the IgE-binding patterns of 3 different patients (A, B, and C) suffering from the celery-mugwort-birch-spice syndrome and tested on 1) the Apiaceae spices anise, fennel, coriander, and cumin, 2) mugwort pollen, celery, and 3) nApi g 5 and HRP. Note that patients A and B recognized more Api g 5 than HRP, whereas patient C showed the opposite, indicating that carbohydrate decorations of allergens are important for some, but not all patients.
To generate mimotopes for the HMW allergens, biopanning was performed using the monoclonal antibody BIP3 for screening. During biopanning rounds, the total titers of eluted phages increased continuously from 2.4×104 to 6.2×107 (Fig. 2A). In parallel the proportion of clones testing positive in colony screening assay increased from 14 to 44 out of 60 clones randomly picked after each round (Table). Phage clones that showed high binding capacity for BIP3 in colony screening were sequenced. The deduced amino acid sequences (Table) showed that in panning round 3, one clone (CHKLRCDKAIA) dominated, representing 6 out of the 8 sequenced clones. The 6 amino acid sequences were grouped in a phylogenetic tree according to similarity (Fig. 2B). The dominant clone CHKLRCDKAIA as well as 2 additional clones (CKASSCDTGHC and CFFAWRSLPNC) were chosen for further studies.
To prove the mimicry potential of the selected phage clones with the original epitope of BIP3, inhibition studies were performed with human sera. Indeed, pre-incubation of a human birch-pollen specific serum with the 3 selected phage-displayed-mimotopes resulted in specific reduction in IgE binding to the HMW allergens. Incubation of IgE with an unrelated phage clone, the original phage library, or helper phage did not have any inhibitory effect (Fig. 3).
When BALB/c mice were immunized with the BIP3 mimotopes (Fig. 4A), all formed specific IgG to HMW birch pollen allergens (Fig. 4B), except in the mice of groups 2 and 3, as shown in the middle panels in Fig. 4B. The formed IgG had a pattern similar to that of BIP3 (Fig. 1A) or IgE from human patients tested on various cross-reactive extracts of relevance in the celery-mugwort-birch-spice syndrome (Fig. 3, lane 1; and Supplemental Figure 1).
So far, we could show that the BIP3 mimotopes via molecular mimicry induced IgG against birch pollen HMW allergen. Consequently, we next aimed to reveal the molecular target of BIP3. We pooled sera of mice immunized with the dominant mimotope phage clone CHKLRCDKAIA and tested them for IgG binding to purified glycoprotein Api g 5 (of which no birch pollen homologue has been characterized so far), to HRP as a model glycoprotein, or to non-glycosylated control allergen rPhl p 5. IgG of BIP3-mimotope-immunized animals showed strong binding to Api g 5, moderate binding to HRP, and no reactivity to Phl p 5 (Fig. 5A). The induced IgG titers to Api g 5 reached at least 1:500 (Fig. 5B). When the sera of BIP3 mimotope-immunized mice were pre-incubated to blotted spices, mugwort pollen, celery, Api g 5, and HRP, they reduced IgE binding of serum of patient C which was subsequently incubated (Supplemental Figure 1, panel "C inhib"). The results indicate that Api g 5 and its cross-reactive homologues are the major targets of BIP3, with carbohydrates playing only a minor role in its epitope. Furthermore, phage-displayed BIP3 mimotopes were immunogenic and induced specific IgG titers to Api g 5.
Carbohydrate-specific IgE antibodies are mainly seen as complicating factors in allergy diagnosis as their clinical relevance is still debated. These IgE antibodies specific for cross-reactive carbohydrate determinants (CCDs) have a high prevalence as they are found in 15%-30% of allergic patients' sera.242526 Especially in the celery-birch-mugwort spice syndrome,12482728 HMW allergens decorated by carbohydrates, contributing to their resistance, have been characterized in the past.1025 In this clinical cross-reactivity syndrome, up to 50% of IgE is targeting the celery glycoprotein Api g 51719 of the berberine bridge enzyme-like protein family, of which so far only a grass pollen homologue Phl p 4,14 but none from birch or Apiaceae spices, has been cloned. Our in silico attempts to localize the epitope by structural alignments failed. In accordance with previous work,17 we could show in this study that BIP3 type antibodies react to HMW-allergens in these allergen sources. Our data also suggest that their targets are Api g 5 and its cross-reactive homologues, and that carbohydrates play a minor role in this binding.
Carbohydrate-specific IgE antibodies are prevalent in 15%-30% of allergic patients' sera.242526 In general, they represent a mixture of N-(asparagine)-linked oligosaccharides containing β-1,2-xylose and core α-1,3-fucose29 and belong to the most abundant environmental immune determinants. On the one hand, anti-CCD IgEs have been associated with false positive diagnostic results,30,31 but on the other hand, anaphylactic reactions associated with IgE specific for carbohydrate oligosaccharides, such as galactose-α-1,3-galactose (α-Gal), have been reported.323334 It has also been shown for tomato β-fructofuranosidase Sol a 12 (previously Lyc e 2) possessing multiple carbohydrate moieties, that the glycosylated, but not the deglycosylated protein was able to induce histamine release in mast cells passively sensitized with sera of tomato allergic CCD reactive patients.35 Notably, the affinity of anti-CCD IgE may reach a dissociation constant of 10-10 M/L.36
Our study hypothesized that the BIP3 mimotopes, as epitope surrogates independent of protein-, carbohydrate-, or mixed-nature of the original antigen, should allow bypassing of the uncertain aspects of carbohydrate moieties by translating the epitope into peptides. Active immunotherapy with peptide mimotopes may be of great advantage as they induce blocking IgG, but do not activate inflammatory allergen-specific T cells.373839 Indeed, the mouse immunization studies have demonstrated that the BIP3 mimotopes are immunogenic and that the induced IgG is directed towards allergens relevant for human IgE binding in the celery-birch-mugwort-spice syndrome. This result was supported by the fact that incubation with the mimotopes could substantially decrease IgE binding to the HMW birch pollen proteins.
In the celery-mugwort-birch-spice syndrome, celery has been recognized as a central allergen. The celery allergen Api g 5 consists of a mixture of 2 polypeptides with molecular weights of 53 and 57 kDa, carrying N-glycans of the MUXF3 type, resembling the HMW-allergens relevant in the clinical syndrome. The BIP3 mimotopes induced IgG binding to Api g 5, identifying this allergen as the major target of BIP3 and IgE specific for HMW. Carbohydrates contribute to the Api g 5 IgE epitope, and a recent study showed excellent correlation between IgE reactivity against Api g 5 and MUXF3 (Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc).40 However, the minimal binding of the induced mouse IgG to model glycoprotein HRP suggests that the BIP3 mimotopes predominantly mimic a protein epitope.
Taken together, we report here the generation of peptide mimetics of a cross-reactive Api g 5 epitope relevant in the celery-mugwort-birch-spice syndrome and propose them as candidates for epitope-specific immunotherapy within this clinical syndrome.
Figures and Tables
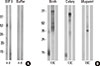 | Fig. 1Binding characteristics of the monoclonal antibody BIP3 on birch pollen extract and cross-reactive allergens Panel A BIP 3 recognizes higher molecular weight proteins in blotted birch pollen extract, which, to a great part, is abrogated after periodate deglycosylation. Lanes n, the untreated extracts; d, birch pollen extract after periodate treatment. Panel B Monoclonal antibodies BIP3 and BIP 1 recognize epitopes in blotted birch pollen extract and cross-reactive allergens of relevance in the celery-mugwort-birch-spice syndrome. BIP1 (lane 1) recognizes Bet v 1 and homologues; BIP3 (lane 3) shows binding to HMW allergens in celery, birch and mugwort pollens. Lanes C, buffer controls. |
 | Fig. 2Increasing phage titers during biopanning indicate enrichment of specific phages; the deduced amino acid sequences of identified phage clones are depicted in a phylogenetic treePanel A: The number of BIP3-eluted phages increased during biopanning from 2.4×104 in the first round to 6.2×107 in the fourth round. Panel B: Phylogenetic tree indicating the degree of similarity between the selected peptides. Clones used for further analysis are marked by * |
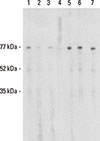 | Fig. 3Selected mimotope clones inhibit human IgE binding to birch pollen HMW allergen. A birch pollen reactive human serum was used either untreated or pre-incubated using 107 phage particles before application to blotted birch pollen extract. Lane 1, no pre-incubation; lane 2, 1-12-cyclo-CKASSCDTGHC; lane 3, 1-12-cylo-CFFAWRSLPNC; lane 4, 1-6-cyclo-CHKLRCDKAIA; lane 5, phage of unrelated specificity, (CRQTRTRTMPGCG); lane 6, original phage library; lane 7, helper phage. |
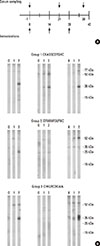 | Fig. 4Immunizations with phage-displayed mimotopes induce BIP3-type IgG in BALB/c micePanel A: Immunization schedule for the 3 selected phage clones. Panel B: IgG reactivities of 3 mice of each group immunized with different BIP3-specific phage clones on blotted birch pollen extract. Group 1, mimotope sequence CKASSCDTGHC; group 2, CFFAWRSLPNC; group 3, CHKLRCDKAIA. Lane 0, pre-immune serum; lane 1, the first immune serum from day 21; lane 2, the second immune serum from day 38. |
 | Fig. 5Identification of Api g 5 as the BIP3 target antigen by mimotope induced immune sera and titer determination. (A) The IgG reactivity of pre-immune (PIS) and the second immune sera (MIS) of mice immunized with the dominant BIP3 mimotope clone CHKLRCDKAIA is shown. y-axis indicating relative binding intensity in ELISA coated with different antigens: glycosylated celery allergen Api g 5 (black bars), non-glycosylated grass pollen allergen Phl p 5 (grey), and weak reactivity to model-glycoprotein HRP (white). (B) Sera of the mice were pooled at equal parts and diluted as indicated on the x-axis before testing on coated Api g 5 in ELISA. Bound IgG (black columns, immune sera; white columns, pre-immune sera) was detected by HRP-labeled anti-mouse IgG. The y-axis indicates the OD values determined at 405 nm. |
ACKNOWLEDGMENTS
The authors thank Dirk Neumann (Scientific Consilience, Saarbrücken, Germany) and Thomas Stockner (Institute of Pharmacology, Medical University Vienna, Austria) for scientific discussions. This study was supported by grants SFB F4606-B19 and F4606-B13 of the Austrian Science Fund.
References
1. Jensen-Jarolim E, Leitner A, Hirschwehr R, Kraft D, Wüthrich B, Scheiner O, et al. Characterization of allergens in Apiaceae spices: anise, fennel, coriander and cumin. Clin Exp Allergy. 1997; 27:1299–1306.
2. Vallier P, Dechamp C, Vial O, Deviller P. A study of allergens in celery with cross-sensitivity to mugwort and birch pollens. Clin Allergy. 1988; 18:491–500.
3. Wüthrich B, Dietschi R. The celery-carrot-mugwort-condiment syndrome: skin test and RAST results. Schweiz Med Wochenschr. 1985; 115:258–264.
4. Wüthrich B, Stäger J, Johansson SG. Celery allergy associated with birch and mugwort pollinosis. Allergy. 1990; 45:566–571.
5. Jarolim E, Tejkl M, Rohac M, Schlerka G, Scheiner O, Kraft D, et al. Monoclonal antibodies against birch pollen allergens: characterization by immunoblotting and use for single-step affinity purification of the major allergen Bet v I. Int Arch Allergy Appl Immunol. 1989; 90:54–60.
6. Fiocchi A, Dahdah L, Martelli A, Mazzina O, Manzotti G. Spice allergies in children. Ann Allergy Asthma Immunol. 2014; 112:72–73.
7. Asero R. Reply to the letter 'multiple nonsteroidal anti-inflammatory drug-induced cutaneous disease: relevance, natural evolution and relationship with atopy' by blanca-lópez et Al. Int Arch Allergy Immunol. 2014; 164:149–150.
8. André F, André C, Colin L, Cacaraci F, Cavagna S. Role of new allergens and of allergens consumption in the increased incidence of food sensitizations in France. Toxicology. 1994; 93:77–83.
9. Asero R. Relevance of pollen-specific IgE levels to the development of Apiaceae hypersensitivity in patients with birch pollen allergy. Allergy. 1997; 52:560–564.
10. Ballmer-Weber BK, Vieths S, Lüttkopf D, Heuschmann P, Wüthrich B. Celery allergy confirmed by double-blind, placebo-controlled food challenge: a clinical study in 32 subjects with a history of adverse reactions to celery root. J Allergy Clin Immunol. 2000; 106:373–378.
11. Scheurer S, Wangorsch A, Haustein D, Vieths S. Cloning of the minor allergen Api g 4 profilin from celery (Apium graveolens) and its cross-reactivity with birch pollen profilin Bet v 2. Clin Exp Allergy. 2000; 30:962–971.
12. Pastorello EA, Farioli L, Stafylaraki C, Scibilia J, Giuffrida MG, Mascheri A, et al. Fennel allergy is a lipid-transfer protein (LTP)-related food hypersensitivity associated with peach allergy. J Agric Food Chem. 2013; 61:740–746.
13. Vejvar E, Himly M, Briza P, Eichhorn S, Ebner C, Hemmer W, et al. Allergenic relevance of nonspecific lipid transfer proteins 2: Identification and characterization of Api g 6 from celery tuber as representative of a novel IgE-binding protein family. Mol Nutr Food Res. 2013; 57:2061–2070.
14. Dewitt AM, Andersson K, Peltre G, Lidholm J. Cloning, expression and immunological characterization of full-length timothy grass pollen allergen Phl p 4, a berberine bridge enzyme-like protein with homology to celery allergen Api g 5. Clin Exp Allergy. 2006; 36:77–86.
15. Fötisch K, Altmann F, Haustein D, Vieths S. Involvement of carbohydrate epitopes in the IgE response of celery-allergic patients. Int Arch Allergy Immunol. 1999; 120:30–42.
16. Borghesan F, Mistrello G, Amato S, Giuffrida MG, Villalta D, Asero R. Mugwort-fennel-allergy-syndrome associated with sensitization to an allergen homologous to Api g 5. Eur Ann Allergy Clin Immunol. 2013; 45:130–137.
17. Bublin M, Radauer C, Wilson IB, Kraft D, Scheiner O, Breiteneder H, et al. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J. 2003; 17:1697–1699.
18. Ganglberger E, Radauer C, Grimm R, Hoffmann-Sommergruber K, Breiteneder H, Scheiner O, et al. N-terminal sequences of high molecular weight allergens from celery tuber. Clin Exp Allergy. 2000; 30:566–570.
19. Bublin M, Lauer I, Oberhuber C, Alessandri S, Briza P, Radauer C, et al. Production and characterization of an allergen panel for component-resolved diagnosis of celery allergy. Mol Nutr Food Res. 2008; 52:Suppl 2. S241–S250.
20. Jensen-jarolim E, Leitner A, Kalchhauser H, Zürcher A, Ganglberger E, Bohle B, et al. Peptide mimotopes displayed by phage inhibit antibody binding to bet v 1, the major birch pollen allergen, and induce specific IgG response in mice. FASEB J. 1998; 12:1635–1642.
21. Wuhrer M, Balog CI, Koeleman CA, Deelder AM, Hokke CH. New features of site-specific horseradish peroxidase (HRP) glycosylation uncovered by nano-LC-MS with repeated ion-isolation/fragmentation cycles. Biochim Biophys Acta. 2005; 1723:229–239.
22. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.
23. Luzzago A, Felici F, Tramontano A, Pessi A, Cortese R. Mimicking of discontinuous epitopes by phage-displayed peptides, I. Epitope mapping of human H ferritin using a phage library of constrained peptides. Gene. 1993; 128:51–57.
24. Aalberse RC, van Ree R. Crossreactive carbohydrate determinants. Clin Rev Allergy Immunol. 1997; 15:375–387.
25. Ballmer-Weber BK, Hoffmann A, Wüthrich B, Lüttkopf D, Pompei C, Wangorsch A, et al. Influence of food processing on the allergenicity of celery: DBPCFC with celery spice and cooked celery in patients with celery allergy. Allergy. 2002; 57:228–235.
26. Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002; 129:286–295.
27. Helbling A, Lopez M, Schwartz HJ, Lehrer SB. Reactivity of carrot-specific IgE antibodies with celery, apiaceous spices, and birch pollen. Ann Allergy. 1993; 70:495–499.
28. Stäger J, Wüthrich B, Johansson SG. Spice allergy in celery-sensitive patients. Allergy. 1991; 46:475–478.
29. Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007; 142:99–115.
30. Mari A, Iacovacci P, Afferni C, Barletta B, Tinghino R, Di Felice G, et al. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergic diseases. J Allergy Clin Immunol. 1999; 103:1005–1011.
31. van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997; 100:327–334.
32. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008; 358:1109–1117.
33. Commins SP, Platts-Mills TA. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J Allergy Clin Immunol. 2009; 124:652–657.
34. Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007; 364:8–18.
35. Foetisch K, Westphal S, Lauer I, Retzek M, Altmann F, Kolarich D, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003; 111:889–896.
36. Jin C, Hantusch B, Hemmer W, Stadlmann J, Altmann F. Affinity of IgE and IgG against cross-reactive carbohydrate determinants on plant and insect glycoproteins. J Allergy Clin Immunol. 2008; 121:185–190.e2.
37. Schöll I, Wiedermann U, Förster-Waldl E, Ganglberger E, Baier K, Boltz-Nitulescu G, et al. Phage-displayed Bet mim 1, a mimotope of the major birch pollen allergen Bet v 1, induces B cell responses to the natural antigen using bystander T cell help. Clin Exp Allergy. 2002; 32:1583–1588.
38. Wallmann J, Epstein MM, Singh P, Brunner R, Szalai K, El-Housseiny L, et al. Mimotope vaccination for therapy of allergic asthma: anti-inflammatory effects in a mouse model. Clin Exp Allergy. 2010; 40:650–658.
39. Pacios LF, Tordesillas L, Cuesta-Herranz J, Compes E, Sánchez-Monge R, Palacín A, et al. Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: peach Pru p 3 allergen as a model. Mol Immunol. 2008; 45:2269–2276.
40. Bauermeister K, Ballmer-Weber BK, Bublin M, Fritsche P, Hanschmann KM, Hoffmann-Sommergruber K, et al. Assessment of component-resolved in vitro diagnosis of celeriac allergy. J Allergy Clin Immunol. 2009; 124:1273–1281.e2.
Supplementary Material
Supplemental Figure 1
IgE binding to allergens relevant in the celery-mugwort-birch-spice syndrome. Extracts and proteins were separated on the same SDS-PAGE gel, blotted, and tested with sera of patients (A), (B), and (C), suffering from this cross-reactivity syndrome. Allergens are indicated on top: An: anise; Fe: fennel; Co: coriander; Cu: cumin; MP: mugwort pollen; Ce: celery; A5: nApi g 5; H: HRP. Panel C inhib: the blot was first pre-incubated with a pool of 3 BIP3-mimotope immunized mice, before incubating with patient C's serum. Bound IgE was detected by peroxidase-labeled anti-IgE. Left: molecular weight markers.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download