Abstract
Purpose
There is increasing epidemiological evidence of an association between childhood obesity and atopic dermatitis, but little is known about the underlying mechanism(s). In the present study, we used a rat model of atopic dermatitis to assess whether juvenile obesity, induced by reduction of litter size, aggravated the signs of atopic dermatitis and, if so, whether this aggravation was associated with changes in plasma concentration of adipokines, such as leptin and adiponectin.
Methods
Dermatitis was induced by neonatal capsaicin treatment. Body weight, dermatitis score, serum IgE, skin nerve growth factor (NGF), serum leptin and adiponectin, and cytokine mRNA expression in the skin lesion were compared between small (SL, 5 pups) and large litters (LL, 15 pups).
Results
The body weight of juvenile rats up to 6 weeks of age was significantly heavier in the SL group, compared with those in the LL group. The SL group showed more robust development of dermatitis, and higher levels of serum IgE and skin NGF than the LL group. Additionally, the SL group demonstrated higher levels of leptin and pro-inflammatory cytokine mRNA but lower levels of adiponectin than the LL group.
In recent decades, the prevalence of atopic dermatitis has increased considerably,1,2 especially among children in well-developed countries.3 The incidence of childhood obesity has also shown a similar trend to atopic dermatitis, leading to suggestions of a causal link between childhood obesity and atopic dermatitis.4 Obesity is known to be implicated in several types of non-communicable diseases, such as metabolic and allergic diseases.5 In fact, there has been accumulating clinical evidence for an association between childhood obesity and atopic dermatitis.6,7,8 However, few conclusive findings have been reported due to possible biases and confounding factors.
Recently, we developed a rat model of atopic dermatitis, produced by neonatal capsaicin treatment.9 Neonatal capsaicin treatment resulted in not only scratching behaviors and pruritic dermatitis, in a relapsing manner, but also pathophysiological changes in the skin, such as a deficiency in epidermal filaggrin expression and increased numbers of mast cells. Additionally, dermatitic rats showed higher levels of serum IgE and skin nerve growth factor (NGF).
The size of the litter in which pups are reared strongly affects their postnatal growth; rat litter size was shown to be inversely related to individual body weight at weaning and throughout adulthood.10 Thus, an artificial reduction in litter size after birth can be used to induce a form of obesity in juvenile rats.
Adipose tissue secretes adipokines, such as leptin and adiponectin. Leptin is known to be upregulated in obesity and associated with inflammation due to its proinflammatory actions. Indeed, serum levels of leptin were associated positively with the prevalence of atopic dermatitis in children11 and adults.12 At the same time, obesity is known to decrease the secretion of adiponectin, a factor with known anti-inflammatory actions.13,14 Interestingly, adiponectin has been known to relieve allergic inflammation and hyperresponsiveness,15 leading to the hypothesis that the reduction in adiponectin caused by obesity could aggravate the signs of atopic dermatitis.
In the present study, we examined whether juvenile obesity, induced by a reduction in litter size aggravated the signs of atopic dermatitis in our rat model9 and, if so, whether this aggravation was associated with changes in serum levels of leptin and adiponectin.
All experiments were approved by the Korea University College of Medicine Animal Research Policies Committee (KUIACUC-2009-134). Pregnant Sprague-Dawley rats were obtained 1 week before parturition and allowed to deliver. Within 6 hours after the birth of the last pup, all pups were removed from their dams and randomly reallocated to form litters of 5 (small litter, SL group) and 15 (large litter, LL group). Following pup reallocation, the litters were individually weighed weekly until 6 weeks after birth. Pups were separated into same-sex littermate pairs and housed in groups of 3 or 4 after weaning at postnatal week 3 (P3W). Only males were used in the present study due to the effects of the estrous cycle on itch behaviors. All animals were reared in a room maintained under a 12:12-hours light/dark cycle (lights on at 07:00 am) and at 22-25℃ with access to food and water ad libitum.
As described previously,9 newborn rat pups were injected subcutaneously within 48 hours of birth with capsaicin (50 mg/kg, Sigma) or vehicle (saline containing 10% Tween 80 and 10% ethanol) at a volume of 10 µL/g body weight.
Cutaneous lesions were compared between the SL and LL groups. Cutaneous lesions were assessed by scoring under gas anesthesia, as described previously.16 Briefly, the skin condition in 3 regions (face, ears, and back) was scored with the following criteria: for face and back, 0 (normal), 1 (wispy fur), 2 (alopecia and flare) or 3 (bleeding or ulcerative lesion) points and for the ears, 0 (normal), 1 (flare), 2 (bleeding) or 3 (loss of part of the ear) points. The sum of the scores in the 3 regions was deemed the dermatitis score for each rat.
Levels of serum IgE, adiponectin and leptin, and NGF in the skin samples were compared between the SL and LL groups. Serum IgE, adiponectin and leptin, and NGF in the skin samples were quantified using a rat IgE ELISA kit (Bethyl Laboratories, Montgomery, USA), a rat leptin ELISA kit (CUSABIO, Wuhan, China), a rat adiponectin ELISA kit (Invitrogen, CA, USA), and a NGF sandwich ELISA kit, ChemiKine (EMD Millipore, MA, USA), respectively, according to the manufacturers' protocols.
Total RNA was extracted from homogenized skin tissue with the TRIzol reagent and reverse-transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen). Quantitative real-time PCR was carried out with SYBR Green in Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, CA, USA), using the following primers: rat IL-1β (forward 5'-CTCCATGAGCTTTGTACAAG-3' and reverse 5'-TGCTGATGTACCAGTTGGGG-3'), IL-4 (forward 5'-CGTGATGTACCTCCGTGCTT-3' and reverse 5'-TTCAGTGTTGTGAGCGTGGA-3'), IL-5 (forward 5'-GAGGGGGCACTGTGGAAATA-3' and reverse 5'-ACTCATCACGCCAAGGAACT-3'), IL-6 (forward 5'-GCCCTTCAGGAACAGCTATG-3' and reverse 5'-CAGAATTGCCATTGCACAAC-3'), IL-10 (forward 5'-CCTGGTAGAAGTGATGCCCC-3' and reverse 5'-TGCTCCACTGCCTTGCTTTT-3'), IL-13 (forward 5'-CCTGGAATCCCTGACCAACA-3' and reverse 5'-GCCATAGCGGAAAAGTTGCT-3'), IL-31 (forward 5'-CTCCAAAGTAGACCTGCCCG-3' and reverse 5'-ACATGGCTAGTTACGACGGC-3'), IL-33 (forward 5'-GCCCTGAGCACATACAACGA-3' and reverse 5'-ACCGTCTCCTGATTGTGCAG-3') and TNF-α (forward 5'-GAAAGTCAGCCTCCTCTCCG-3' and reverse 5'-CTCCAAAGTAGACCTGCCCG-3'). Experiments were conducted in duplicate. Relative expression of each target gene was quantified by the 2-ΔΔCt method, normalizing each gene expression to GAPDH (forward 5'-ACTTTGGCATCGTGGAAGGG-3' and reverse 5'-ACATTGGGGGTAGGAACACG-3'). Data were expressed in arbitrary units, as a fold-increase in the SL group compared with the LL group.
All data are presented as means±SEMs. Statistical significances were analyzed using Student's t-test or the Mann-Whitney rank sum test, depending on the normality test. A difference of P<0.05 was considered to indicate statistical significance. All statistical analyses were carried out using Sigma Stat (ver. 3.5; Systat Software Inc., IL, USA).
To examine whether litter size would affect the body weight, we compared body weights between the SL and LL groups. The rat pups raised in the SL group weighed heavier during a period of P2W to P6W compared with the LL group (Fig. 1A); from P2W to P6W, the average body weight of rat pups in the SL group was consistently over 120% of those in the LL group. From P1W to P3W, body weights were heavier in the SL group than in the LL group (Fig. 1B). These results suggest that litter size is inversely related to body weight.
As we reported previously,9 neonatal capsaicin treatment induced scratching behaviors and pruritic dermatitis characterized by cutaneous lesions, such as alopecia, superficial erosion, deep excoriation, hemorrhages, and scars. Cutaneous lesions were detected primarily around the head, neck, upper forelimbs, and rostral back regions. As illustrated in Fig. 1C, cutaneous lesions in the SL rats first appeared at P4W, while those in the LL animals were not detected until P5W. Additionally, from P4W to P6W, cutaneous lesions were significantly more robust in the SL group than in the LL group, as shown by the consistently higher dermatitis score. At P5W, the number of robust regions was larger in the SL group than in the LL group (Fig. 1D). These results suggest that juvenile obesity may aggravate the signs of atopic dermatitis.
Next, we investigated biochemical changes related to atopic dermatitis, such as serum IgE and skin NGF. At P1W, P2W, P4W, and P.6W, serum IgE levels were significantly higher in the SL group than in the LL group (Fig. 2A). At P1W, P2W, and P4W, The tissue NGF levels of back skin samples were also significantly higher in the SL group than in the LL group (Fig. 2B). These data also suggest that juvenile obesity exacerbates the biochemical signs of atopic dermatitis.
Obesity is known to closely correlate with changes in adipokines, such as leptin, a pro-inflammatory cytokine, and adiponectin, an anti-inflammatory cytokine. From P2W to P6W, serum leptin levels were significantly higher in the SL group than in the LL group (Fig. 3A). During the test period, however, serum adiponectin levels were significantly lower in the SL group than in the LL group (Fig. 3B). Taken together, juvenile obesity was paralleled by changes in adipokines that may aggravate the signs of atopic dermatitis.
Finally, we examined whether the upregulation of serum leptin associated with obesity would affect Th1/Th2 cytokine mRNA expression in the skin lesion of atopic dermatitis. Most cytokine mRNAs were markedly overexpressed in the SL group, when compared with the LL group (P<0.001, Mann-Whitney rank sum test)(Fig. 4). In particular, TNF-α, IL-13, and IL-31 mRNA levels were more than 10-fold higher in the SL group than in the LL group. However, expression of mRNA for IL-4, the hallmark Th2 cytokine, in the SL group was downregulated by 46%, compared with the LL group (P<0.01, Mann-Whitney rank sum test). There was no significant difference in IL-5 or IL-10 mRNA expression between the groups. Given the elevated levels of serum leptin and IgE (Fig. 3), higher expressions of pro-inflammatory cytokines, such as IL-1β, TNF-α, IL-6, IL-31, and IL-33, in the SL group indicates that leptin promotes immune and inflammatory responses, leading to a worsening of atopic dermatitis.
Using a rat model of atopic dermatitis, induced by neonatal capsaicin treatment, we showed that rats raised in small litters (the SL group) had heavier body weights and more severe dermatitis, as well as higher levels of serum IgE and skin NGF than rats raised in larger litters (the LL group). Additionally, the SL group demonstrated higher serum levels of leptin and lower serum levels of adiponectin than the LL group. To our knowledge, this is the first reported experimental study demonstrating a link between juvenile obesity and aggravation of the signs of atopic dermatitis.
Modifying the sizes of litters during the preweaning period is known to affect the growth of the pups.17,18 Animals raised in litters of 16 have significantly lower body weight than any smaller litter size (2 to 12).10 The results of this study also demonstrate that rat pups in the SL group (5 pups) weighed heavier during a period of P2W to P6W than the LL group (15 pups). The difference in body weight is presumably due to the higher amount of breast milk available to the pups in the SL group vs the LL group. Childhood body weight in excess of 110% or 120% of age-matched normal controls is categorized as overweight or obese, respectively.19 In the present study, the body weight of rat pups in the SL group was over 120% of those in the LL group from P2W to P6W; thus, following the criteria for children, we rated these rats as obese.
A high prevalence of atopic dermatitis has been reported in Europe and North America, as well as a striking increase in the prevalence in developing countries too.20 To date, the cause(s) of this increase remain(s) unclear. Given that the prevalence of childhood obesity increased dramatically in most industrialized countries around the same time,21 obesity has been hypothesized to increase the prevalence of atopic conditions.22 However, while obesity seems to be a risk factor for asthma,23,24,25,26,27,28 few reports had demonstrated a positive relationship between obesity and other atopic conditions, such as allergic rhinitis and atopic dermatitis. Recently, several lines of clinical evidence have been reported that obesity in children is associated with an increased risk of atopic dermatitis.6,7,29 In the present study, we also demonstrated that obese rats in the SL group showed more severe atopic dermatitis signs than the non-obese animals of the LL group.
Obesity induces dysregulation of adipokines such as leptin and adiponectin, leading to a low-grade chronic inflammation, known as metainflammation.30 This, in turn, is thought to be associated with the pathogenesis of atopy through altered inflammatory or immune responses (i.e., immunological tolerance).22 Leptin is structurally similar to pro-inflammatory cytokines, such as IL-2 and growth hormone 1, and thus may possess a pro-inflammatory action.22 In fact, leptin has been shown to increase the expression of pro-inflammatory cytokines, especially TNF-α and IL-6, in many types of cells including adipocytes, macrophages and T lymphocytes.31 An increase in leptin with obesity promotes the secretion of TNF-α, which, in turn, leads to increases in other pro-inflammatory cytokines such as IL-6 and TNF-α and reductions in anti-inflammatory cytokines, adiponectin.32 Consistently, here, we showed that mRNA levels of pro-inflammatory cytokines in the skin lesions, including TNF-α and IL-6, which have been reported to be implicated in the pathogenesis of atopic dermatitis, were much higher in the obese atopic rats than those in the non-obese ones (Fig. 4), suggesting that leptin may be linked to worsening inflammatory skin condition in the obese atopic rats. Leptin could also induce allergic inflammation by activation of eosinophils via an altered expression profile of chemokines and cytokines, and chemokinesis.33 Thus, the serum level of leptin seems to be positively related to atopic dermatitis through its pro-inflammatory actions.11,12
The present results demonstrate that obese rats in the SL group showing higher serum levels of leptin exhibited more severe signs of atopic dermatitis. Notably, the levels of serum IgE and skin NGF between the 2 groups were clearly differentiated for up to 2 weeks following neonatal capsaicin treatment, prior to the induction of macroscopic dermatitis, suggesting that these disparities could be a cause, not a consequence, of the difference in atopic dermatitis. In fact, the incidence of specific IgE positivity was 3 times higher in the obese group than in the non-obese group.34 Furthermore, the positive correlations have been shown between serum levels of leptin and IgE, and between body mass index and atopic sensitization.35,36
In contrast with leptin, several reports have demonstrated a negative correlation between the levels of adiponectin and pro-inflammatory markers, such as C reactive protein and tumor necrosis factor-α in metabolic diseases.37,38,39 Additionally, adiponectin could relieve allergic inflammation and hyperresponsiveness,13,14 and, intriguingly, low plasma adiponectin levels in obese children have been associated with an increased prevalence of atopic dermatitis.6 Consistently, in the present study, obese rats in the SL group demonstrated lower serum levels of adiponectin than the animals in the LL group. Together with previous reports, the decrease in immunological tolerance to antigens induced by juvenile obesity via the changes in leptin and adiponectin may play an important role in exacerbation of atopic dermatitis.
Studies in full-term human infants have shown that exclusive breastfeeding for at least 3 months, in fact, reduced the risk of atopic dermatitis compared with feeding cow milk in children with a family history of atopic dermatitis.40 This suggests that there is likely to be a considerable protective effect of breast milk against atopic dermatitis. However, in the present study, rat pups in the SL group, which were overfed breast milk, suffered from more severe signs of atopic dermatitis than those in the LL group, which fed on a more limited amount of breast milk. Thus, although breast milk may confer protective effects against atopic dermatitis versus non-maternal milk, juvenile obesity induced by the overfeeding of maternal milk could exacerbate the signs of existing atopic dermatitis.
In conclusion, the decrease in immunological tolerance, evidenced by the increase in serum leptin and the decrease in adiponectin induced by juvenile obesity may be related to the aggravation of atopic dermatitis.
Figures and Tables
Fig. 1
Relationship between obesity and atopic dermatitis. (A) Body weight of pups reared in litters of 5 (small litters, SL group) and 15 (large litters, LL group). The body weights of the rats were compared between the SL and the LL groups from 1 to 6 weeks after neonatal capsaicin treatment. Data indicate means±SEM. **P < 0.01, ***P < 0.001 (t-test), vs the LL group. (B) Comparison of body size between the SL and LL groups at 1, 2, and 3 weeks after neonatal capsaicin treatment. (C) Dermatitis score of pups reared in the SL and LL groups. The dermatitis score was compared between the SL and the LL groups from 1 to 6 weeks after neonatal capsaicin treatment. Data indicate means±SEM. ***P < 0.001 (t-test), vs the LL group. (D) Comparison of cutaneous lesions between the SL and LL groups at 5 weeks after neonatal capsaicin treatment.

Fig. 2
Serum IgE (A) and skin NGF (B) of the SL and LL groups. Hyperproduction of serum IgE and skin NGF was observed in the SL group suffering from severe dermatitis vs the LL group. Data indicate means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (t-test), vs the LL group.
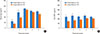
Fig. 3
Serum leptin (A) and adiponectin (B) in the SL and LL groups. Increases and decreases in leptin and adiponectin, respectively, were observed in the SL group showing more severe dermatitis than the LL group. Data indicate means ± SEM. **P < 0.01, ***P < 0.001 (t-test), vs the LL group.

Fig. 4
Expression of cytokine mRNA in the skin lesion of the SL and LL rats at 5 weeks of age. IL-1β, TNF-β, IL-6, IL-13, IL-31 and IL-33 mRNA levels were significantly higher in the SL group than in the LL group, whereas IL4 mRNA levels were lower in the SL group. There was no significant difference in IL-5 or IL-10 mRNA levels between the groups. Data are expressed in arbitrary units, as a fold-increase vs the LL group. **P < 0.01, ***P < 0.001 (Mann-Whitney rank sum test).

ACKNOWLEDGMENTS
We thank Alexander J. Davies for editorial assistance. This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012-0009675 and NRF-2013R1A1A2011913) and a Korea University Grant.
References
1. Eichenfield LF, Hanifin JM, Beck LA, Lemanske RF Jr, Sampson HA, Weiss ST, Leung DY. Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics. 2003; 111:608–616.
2. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H. ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006; 368:733–743.
3. Maziak W, Behrens T, Brasky TM, Duhme H, Rzehak P, Weiland SK, Keil U. Are asthma and allergies in children and adolescents increasing? Results from ISAAC phase I and phase III surveys in Münster, Germany. Allergy. 2003; 58:572–579.
4. Rokholm B, Baker JL, Sørensen TI. The levelling off of the obesity epidemic since the year 1999--a review of evidence and perspectives. Obes Rev. 2010; 11:835–846.
5. Kundi M. Obesity and atopy: what is the relationship? Int Arch Allergy Immunol. 2010; 153:321–322.
6. Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009; 20:81–88.
7. Machura E, Szczepanska M, Ziora K, Ziora D, Swietochowska E, Barc-Czarnecka M, Kasperska-Zajac A. Evaluation of adipokines: apelin, visfatin, and resistin in children with atopic dermatitis. Mediators Inflamm. 2013; 2013:760691.
8. Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007; 62:1205–1213.
9. Back SK, Jeong KY, Li C, Lee J, Lee SB, Na HS. Chronically relapsing pruritic dermatitis in the rats treated as neonate with capsaicin; a potential rat model of human atopic dermatitis. J Dermatol Sci. 2012; 67:111–119.
10. Wurtman JJ, Miller SA. Effect of litter size on weight gain in rats. J Nutr. 1976; 106:697–701.
11. Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004; 114:254–259.
12. Sood A, Ford ES, Camargo CA Jr. Association between leptin and asthma in adults. Thorax. 2006; 61:300–305.
13. Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, Shirahata A, Taniyama M. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res. 2003; 11:1072–1079.
14. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004; 350:2362–2374.
15. Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006; 118:389–395.
16. Mihara K, Kuratani K, Matsui T, Nakamura M, Yokota K. Vital role of the itch-scratch response in development of spontaneous dermatitis in NC/Nga mice. Br J Dermatol. 2004; 151:335–345.
17. Widdowson EM, Mc CR. Some effects of accelerating growth. I. General somatic development. Proc R Soc Lond B Biol Sci. 1960; 152:188–206.
18. Knittle JL, Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968; 47:2091–2098.
19. Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006; 1:11–25.
20. Wüthrich B. Clinical aspects, epidemiology, and prognosis of atopic dermatitis. Ann Allergy Asthma Immunol. 1999; 83:464–470.
21. Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010; 375:1737–1748.
22. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11:85–97.
23. Kim SH, Sutherland ER, Gelfand EW. Is there a link between obesity and asthma? Allergy Asthma Immunol Res. 2014; 6:189–195.
24. Chen Y, Dales R, Krewski D, Breithaupt K. Increased effects of smoking and obesity on asthma among female Canadians: the National Population Health Survey, 1994-1995. Am J Epidemiol. 1999; 150:255–262.
25. Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002; 122:1256–1263.
26. Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999; 54:396–402.
27. Cassol VE, Rizzato TM, Teche SP, Basso DF, Centenaro DF, Maldonado M, Moraes EZ, Hirakata VN, Solé D, Menna-Barreto SS. Obesity and its relationship with asthma prevalence and severity in adolescents from southern Brazil. J Asthma. 2006; 43:57–60.
28. Bråbäck L, Hjern A, Rasmussen F. Body mass index, asthma and allergic rhinoconjunctivitis in Swedish conscripts-a national cohort study over three decades. Respir Med. 2005; 99:1010–1014.
29. Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu JH, Kim BJ, Hong SJ. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int Arch Allergy Immunol. 2011; 154:42–48.
30. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011; 121:2111–2117.
31. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005; 115:911–919.
32. Wang B, Trayhurn P. Acute and prolonged effects of TNF-α on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflugers Arch. 2006; 452:418–427.
33. Wong CK, Cheung PF, Lam CW. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur J Immunol. 2007; 37:2337–2348.
34. Vieira VJ, Ronan AM, Windt MR, Tagliaferro AR. Elevated atopy in healthy obese women. Am J Clin Nutr. 2005; 82:504–509.
35. Xu B, Jarvelin MR, Pekkanen J. Body build and atopy. J Allergy Clin Immunol. 2000; 105:393–394.
36. Porter M, Wegienka G, Havstad S, Nageotte CG, Johnson CC, Ownby DR, Zoratti EM. Relationship between childhood body mass index and young adult asthma. Ann Allergy Asthma Immunol. 2012; 109:408–411.e1.
37. Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003; 14:561–566.
38. Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010; 120:3466–3479.
39. Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003; 107:671–674.
40. Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007; 1–186.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download