Abstract
Ovomucoid (OMC) is the most prominent allergen causing hen's egg allergy, containing disulfide (S-S) bonds that may be responsible for its allergic action. As S-S bonds may be reduced during electrolysis, this study was undertaken to evaluate modulation of the allergic action of OMC after electrolysis. Electrolysis was carried out for 1% OMC containing 1% sodium chloride for 30 minutes with a voltage difference of 90 V, 0.23 A (30 mA/cm2). Protein assays, amino acid measurement, and mass spectrometry in untreated OMC and OMC on both the anode and cathode sides after electrolysis were performed. Moreover, 21 patients with IgE-mediated hen's egg allergy were evaluated by using the skin prick test (SPT) for untreated OMC and OMC after electrolysis. The allergic action of OMC was reduced after electrolysis on both the anode and cathode sides when evaluated by the SPT. The modifications of OMC on electrolysis caused the loss of 2 distinct peptide fragments (57E-63K and 123H-128R) as seen on matrix-associated laser desorption/ionization time-of-flight mass spectrometry. The total free SH groups in OMC were increased on the cathode side. Although the regions of S-S broken bonds were not determined in this study, the change in S-S bonds in OMC on both the anode and cathode sides may reduce the allergenic activity.
Patients with food allergies have been increasing in recent years, 5%-10% of infants and 1%-3% of schoolage child in Japan being affected by food allergies.1 The most common causative food is hen's eggs, which have been reported to account for 30% of food allergy cases in Japan.2 Patients with hen's egg allergy develop an adverse reaction after ingestion of eggs or egg products, namely, urticaria, angioedema, wheezing, gastrointestinal symptoms, or anaphylactic shock. Hen's egg allergens are mainly included in the egg white. Ovomucoid (OMC) has been identified as the most prominent allergen causing hen's egg allergy and contains S-S bonds that may be responsible for its allergic action.34
OMC is a globular protein composed of 186 amino acids and comprises Ga1 d 1.1, Ga1 d 1.2, and Ga1 d 1.3.5 Ga1 d 1.1 has 1 α-helix plus 3 β-sheet conformation. The α-helix conformation disintegrates, and the allergenic activity of OMC significantly decreases when phenylalanine, the 37th amino acid in OMC, is substituted with methionine.6 Because the allergenic activity of OMC cannot be decreased by heating,7 attenuation of this allergenic activity is an important issue in medicine. A trial in which OMC was removed from the egg white and some methods by which the allergenic activity of OMC was mitigated have been reported.568
We reported that the allergenic activity of β-lactoglobulin (BLG), which is the most prominent S-S protein allergen as well as the main whey allergen, was markedly mitigated by electrolysis.9 This phenomenon is understood to be due to immunogenicity attenuated by a change in the higher order structure of BLG. OMC is also a protein with a solid higher order structure, and it has been reported that a change in this higher order structure leads to mitigation of the allergenic activity of OMC.5
In this study, we aimed to determine whether or not electrolysis could change the higher order structure of OMC and mitigate its allergenic activity. The skin prick test (SPT) was performed on children with hen's egg allergy for the evaluation of the allergenic activity of OMC after electrolysis. This study may offer a new strategy for egg products in patients with hen's egg allergy.
Twenty-one patients with IgE-mediated hen's egg allergy were enrolled in this study. All of them exhibited a previous adverse reaction to hen's eggs or hen's egg-containing food, namely, urticaria/angiedema (n=10), wheezing (n=6), gastrointestinal symptoms (n=2), anaphylactic shock (n=0), skin symptoms plus wheezing (n=2), or skin symptoms plus wheezing, and gastrointestinal symptoms (n=1). The immediate adverse reactions were confirmed by oral provocation tests with boiled hen's eggs in all patients.
The study was approved by the Human Research Advisory Board, and written informed consent for the investigation was obtained from all the study participants, the parents'consent being obtained for children.
The SPT was performed with plastic bifurcated needles (DUOTIP-TEST; Lincoln Diagnostics, Decatur, IL, USA), and the diameters of the wheals (mm) were determined after 20 minutes. Normal saline with or without 1% histamine hydrochloride was applied as a control. The test was performed for untreated OMC and OMC after electrolysis. Each solution was placed at random on the forearm. Purified OMC, 10,000 BAEE µ/mg trypsin inhibitor, was obtained from Nacalai Tesque Inc., Kyoto, Japan.
Heat-tight, 30-mm bore glass tubes were used in an H-shaped electrolysis cell, connected with 2 tubes of 300 mm length and 1 tube of 40 mm length (Fig. 1A). To collect samples during electrolysis, a 5-mm tap-equipped bore glass tube was inserted through a rubber gasket into each platinum electrode 15 mm wide and 30 mm long (Fig. 1B). In addition, 5-mm bore silicon tubes were coiled around the electrolysis cell, circulating -5℃ water originating from a thermostated water bath (Model TBF210DA; Advantec MFS, Tokyo, Japan) (Fig. 1C). Electrolysis was carried out for 1% OMC containing 1% sodium chloride for 30 minutes with a voltage difference of 90 V, 0.23 A (30 mA/cm2). An electrolyte volume of 260 mL was used. The temperature in the electrolyte in both glass tubes was kept in the range of 12℃-18℃, the temperature next to the electrodes ranging from 40℃ to 50℃ during the electrolysis. Each OMC solution was then filtered through a 0.45-mm filter unit (Millex-HA; Millipore, Bedford, MA, USA). To confirm the stability, samples were kept at 4℃ for more than 2 weeks in sterilized polystyrene tubes (Falcon 2054; Beckton-Dickinson, Franklin Lakes, NJ, USA) prior to tests.
The amounts of protein were determined by the method of Lowry et al.10 using bovine serum albumin as the standard protein. The redox potential of the samples was measured with a redox electrode (model HM-21P; DKK-TOA Co., Tokyo, Japan) calibrated with a standard potassium chloride solution at 210 mV. Free SH groups were determined by quantifying total SH groups according to Ellman's method.11 The total amino acid composition was determined using a high-performance liquid chromatograph (HPLC) for amino acid analysis (model L-850; Hitachi, Ibaragi, Japan) after incubation of the samples with the same volume of 12 N HCl for 20 hours at 100℃.
After dissolving OMC in a lysis buffer (7 M urea, 2 M thiourea, 2% CHAPS, and 40-mm Tris), 2-dimensional polyacrylamide gel electrophoresis (2D-PAGE) was performed with 5-mm TATA-binding protein and 10-mm acrylamide applied per isoelectric focusing gel, a pl 3-10. Isoelectric focusing was performed until about 35,000 volt-hours, and the proteins separated on 2D-PAGE were visualized by rapid Coomassie staining. Some spots were cut out from the gel, and tryptic digestion was then performed simultaneously according to the procedure of Gevaert et al.12 A peptide mass fingerprint database search was performed with the masses obtained by means of MALDI-ToF-MS (Shimadzu Biotech, Kyoto, Japan), with a 337-nm nitrogen laser (Kratos Analytical, Manchester, UK) in a positive ion mode. The monoisotopic m/z values of tryptic peptide peaks were entered into Mascot (Matrix Science, http://www.matrixscience.com/search_form_select.html) for a database search for the characterization of the proteins.
Representative SPT results for patients with hen's egg allergy are shown in Fig. 2, and the SPT results for 21 patients with hen's egg allergy are presented in Table 1. The wheal diameter for OMC on the polar opposites was found to be reduced compared to that for untreated OMC. The percentages of the wheal reaction with OMC on the cathode (cOMC) and anode (aOMC) sides compared to untreated OMC (uOMC) were 77.6%±28.8% and 82.6%±33.8% (P=0.004 vs P=0.030) , respectively.
The pH and redox potential results for the OMC solutions were 5.9 m and +242 mV before electrolysis, but 2.8 m and +302 mV on the anode side and 11.2 m and -15 mV on the cathode side after electrolysis. The OMC concentration was 5.8 mg/mL before electrolysis, which changed to 6.1 mg/mL on the anode side and to 9.4 mg/mL on the cathode side in electrolysis. The quantity of SH groups in OMC was 1.2 nmol/mL before electrolysis, which was much increased on electrolysis on the cathode side (14.8 nmol/mL) but did not change significantly on the anode side (2.8 nmol/mL).
The total amino acid composition of OMC changed equally after 30-minute electrolysis on both cOMC and aOMC, decreasing cysteine (C) residues from 4% to 3% and tyrosine (Y) residues from 3% to 2% (Table 2).
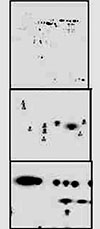
We performed mass spectrometry for uOMC, cOMC, and aOMC after electrolysis, which had been treated with trypsin. As shown in Fig. 3, 2 peptide regions, including cysteine residues (57E-63K and 123H-128R), were not detected in OMC on the cathode or anode side after electrolysis. We presumed that the cysteine residues in these regions underwent some changes on electrolysis. Although we expected that these 2 S-S bonds in cOMC and aOMC were cleaved, the peptide signal on carbamidomethylation was not detected for cOMC and aOMC. Therefore, we suspected that any reactions other than a reduction one were involved in the change of protein structure in the hypoallergenic OMC. Then, we performed 2D electrophoresis with an irreducible solvent for uOMC, cOMC, and aOMC. Extremely diverse spots were obtained (Fig. 4). We cut these relatively large spots out of the 3 respective gels, treated the proteins included in the gels with trypsin, and then performed mass spectrometry for these peptide fragments from the 3 respective gels. Moreover, we calculated the theoretical molecular weight of OMC and compared the theoretical value to the actual one obtained on mass spectrometry. In cOMC, a new formation of S-S bonds in 15E-24K, 57E-82K, and 122R-128R, or 15E-24K, and 165C-185K patterns were expected. In aOMC, a new formation of S-S bonds in 24M-14K, 130E-159K, and 57E-63K, 165C-185K, and 18D-24K, or 90A-121K and 18D-24K patterns were suspected.
OMC containing 186 amino acids is a protein of 28 kDa with an isoelectric point pH of 4.1. OMC consists of almost 60 amino acids each 3 tandem domains which are named Gal d 1.1, Gal d 1.2, and Gal d 1.3, respectively. All these domains have 3 intramolecular S-S bonds but no intermolecular S-S bond.13 OMC is highly resistant to proteolytic enzymes and heat,7 and exhibits high allergenic activity due to this constructional feature. There have been some reports of efforts to obtain hypoallergenic OMC. Cooke and Sampson5 reported that the binding force of OMC to IgE antibodies was decreased by a mean of 28% when its intramolecular S-S bonds were cleaved by dithiothreitol (DDT), this feature being particularly demonstrated in infants with hen's egg allergy. Kato et al.8 reported that the allergenic capacity of OMC disappears when OMC is heated together with wheat protein and becomes aggregated. This phenomenon was attributed to formation of intermolecular S-S bonds between the OMC and the wheat protein, and aggregation of OMC.
When the solid component concentration of egg protein was 0.2%-0.3%, the pH was 10-11.5, and OMC was heated at higher than 80℃, it became denatured and lost its high allergenic capacity.14 Moreover, when OMC was treated at a high pressure of 100-400 MPa, its binding force to IgE antibodies decreased. This is the reason the tyrosine residues of OMC move from the molecular entrails to the molecular surface.
In this study, it was found that the allergenic capacity of OMC was attenuated on energization treatment. This phenomenon for OMC with mitigated allergenicity was evident in the cOMC, and the quantity of SH groups in cOMC significantly increased.
It was expected that some of the 9 S-S bonds in an OMC molecule were cleaved, which is in accord with Cooke and Sampson's report that OMC's ability to bind IgE decreases when the S-S bonds are cleaved by the addition of DDT.5 That study suggested that an intramolecular S-S bond of OMC was cleaved by electric energy without the use of a reducing agent, such as DDT, and that this cleavage of the intramolecular S-S bond contributed to the decreased allergenic capacity. Moreover, on 2D-PAGE, diverse spots were obtained on the uOMC, cOMC, and aOMC gels, and MS spectral analysis of these spots gave various results. Therefore, the S-S bond regions in cOMC and aOMC were likely to have variously changed due to reactions other than the reduction ones, and these changes were considered to contribute to the formation of hypoallergenic OMC.
Figures and Tables
Fig. 1
Pictures of the electrolysis apparatus. (A) Whole image of electrolysis apparatus. (B) Magnified image of the surrounding platinum electrode plates. (C) Cooling system for electrolysis apparatus.

Fig. 3
MALDI-ToF-MS SPECTRA of uOMC (upper), cOMC (middle), and aOMC (lower) after tryptic digestion.

Table 1
The wheal size (mm) on skin prick test in 21 patients with egg allergy
ACKNOWLEDGMENTS
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (KAKENHI No. 22580138) and a Grant-in-Aid for Egg Research from the Kieikai Research Foundation.
References
1. Ebisawa M, Sugizaki C. Prevalence of pediatric allergic diseases in the first 5 years of life. J Allergy Clin Immunol. 2008; 121:S237.
2. Noda R. Prevalence of food allergy in nursery school (nationwide survey). Jpn J Food Allergy. 2010; 10:5–9.
3. Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994; 93:1047–1059.
4. Kovacs-Nolan J, Zhang JW, Hayakawa S, Mine Y. Immunochemical and structural analysis of pepsin-digested egg white ovomucoid. J Agric Food Chem. 2000; 48:6261–6266.
5. Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997; 159:2026–2032.
6. Mine Y, Sasaki E, Zhang JW. Reduction of antigenicity and allergenicity of genetically modified egg white allergen, ovomucoid third domain. Biochem Biophys Res Commun. 2003; 302:133–137.
7. Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997; 100:171–176.
8. Kato Y, Oozawa E, Matsuda T. Decrease in antigenic and allergenic potentials of ovomucoid by heating in the presence of wheat flour: dependence on wheat variety and intermolecular disulfide bridges. J Agric Food Chem. 2001; 49:3661–3665.
9. Matsumoto T. Mitigation of the allergenic activity of beta-lactoglobulin by electrolysis. Pediatr Allergy Immunol. 2011; 22:235–242.
10. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
11. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77.
12. Gevaert K, Verschelde JL, Puype M, Van Damme J, Goethals M, De Boeck S, et al. Structural analysis and identification of gel-purified proteins, available in the femtomole range, using a novel computer program for peptide sequence assignment, by matrix-assisted laser desorption ionization-reflectron time-of-flight-mass spectrometry. Electrophoresis. 1996; 17:918–924.
13. Kato I, Schrode J, Kohr WJ, Laskowski M Jr. Chicken ovomucoid: determination of its amino acid sequence, determination of the trypsin reactive site, and preparation of all three of its domains. Biochemistry. 1987; 26:193–201.
14.
A Hosono
Y Ooi
R Nakamura
. Hypoallergenic egg white and a method of manufacturing hypoallergenic egg white. Japanese patent. P2004-261154A. 2004. Sep. 24.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download