Abstract
Purpose
The production of camel heavy-chain antihuman IgE (huIgE) that has the potential to block IgE-FcεRI interaction and histamine release by basophils.
Methods
Camels were immunized with a synthetic loop peptide (SLP) designed in a multiple antigen peptide system (MAPS) forming SLP-MAPS immunogen. Camel polyclonal antibodies (PCAs) were produced, purified, characterized using Protein A & G, ELISA, and SDS-PAGE, and tested for their potency to block passive sensitization and histamine release of human basophils using flow cytometry (FCM) and ELISA, respectively.
Results
FCM data indicated that camel conventional (IgG1) and heavy chain antibodies (HCAbs; IgG2, and IgG3) had blocking activities of 43.9%, 72%, and 96.6%, respectively. Moreover, both IgG2 and IgG3 achieved remarkable inhibition rates of 93.98% and 97.05% in histamine release, respectively, whereas the IgG1inhibiting activity was 60.05%.
Conclusions
Camel PCAs produced against SLP-MAPS were capable of blocking the IgE-receptor interaction and the release of histamine by basophils with superiority to HCAbs. These findings may pave the way toward the possible use of camel anti-huIgE HCAbs as blocking antibodies in the treatment of IgE-mediated allergy and asthma.
Asthma is a common chronic inflammatory disease that affects the respiratory airways, lungs, and muscles involved in air transport. Several reviews of the epidemiology of asthma have indicated a worldwide increase in prevalence, particularly in the industrial Western countries as well as in developing countries that are undergoing urbanization and industrialization.12 It was estimated that 300 million people worldwide suffer from asthma and that additional 100 million people will become asthmatic by 2025.3
The role of IgE as a reaginic antibody involved in type I hypersensitivity is well established. IgE-C3 binds with high affinity to extracellular α-chain domains of the high-affinity receptor FcεRI.4 Immunotherapy through production of anti-IgE- or anti-Fc receptor-binding site is considered the most specific alternative to chemotherapy and has received intensive research during the last decade.567 Anti-IgE antibodies are IgG antibodies directed against IgE-Cε3 to block the IgE-Fc receptor interaction, particularly IgE-FcεR1. Consequently, they prevent subsequent allergic symptoms.89
In 1993, a novel and unusual class of antibodies was discovered in the serum of camels.10 These antibodies were found to be devoid of both the light chains and the CH1 domains, and thus were named heavy chain antibodies (HCAbs). HCAbs proved to have many unique characteristics attributed to their low molecular weight and single variable domain (VHH).101112 This has led to intensive research toward the production of HCAbs and VHH nanobodies against various viral, bacterial, protozoal, and helminthic parasites, toxins, and tumors as well as other immunologic and functional protein targets.13 VHH were successfully prepared through cloning, expression, and selection of antigen-specific HCAbs and VHHs in bacteria and yeast for potential use in the diagnosis and immunotherapy of various diseases.14151617 As no HCAbs have been produced or characterized against the huIgE-FcεRI binding site, the purpose of this study was to prepare camel HCAbs (IgG2 and IgG3) in addition to conventional IgG1 against the huIgE-FcεRI binding site. Furthermore, these antibodies were assessed for their potency to block binding of huIgE to its FcεRI on human basophils using FCM and to inhibit histamine release from sensitized human basophils.
Immunogen used in camel immunization was a modified synthetic loop peptide (SLP) with the basic sequence CGETYQSRVTHPHLPRALMRSTTKC.18 The SLP was designed as a multiple antigen peptide system (MAPS)19 forming SLP-MAPS immunogen (Alpha Diagnostic International Co., San Antonio, TX, USA). Local male camels (Camelus dromedarius) were immunized with an initial 0.5 mg of SLP-MAPS mixed with Stimmune adjuvant (Prionics, Schlieren-Zurich, Switzerland) at a 1:1 ratio. The camels was immunized 5 times at 2-week intervals, and serum samples were separated from clotted blood and stored at -80℃ for further use.
Camel serum was fractionated by differential absorption on Protein G and Protein A as previously described.10 Protein concentration was determined using Bradford assay,20 and purified isotypes were stored in a BioStab antibody stabilizer (Sigma, St. Louis, MO, USA) at 4℃ and resolved by 12% SDS-PAGE using vertical minigel (Cleaver Scientific, Warwickshire, England). The ELISA was used for the detection of specific anti-SLP-MAPS in immunized camel sera or the purified isotypes using horseradish peroxidase (HRP)-labeled protein A as a tracer. For IgE blocking assay, the protein content of all purified camel IgG isotypes was standardized.
Human basophils were purified from 20 mL of anticoagulated blood of healthy donors using the basophil Isolation Kit II (Miltenyi Biotec, Cologne, Germany). The negatively selected cells were washed by centrifugation, and the final cell pellet was adjusted in 40 µL of HEPES buffer (HE) (127 mM NaCl, 5 mM KCl, 20 mM HEPES, and 5 IU/mL heparin; pH 7.4) to 1.0-1.5×105 cells/test. The cells were stained using the Diff-3 kit (Aromex, Amman, Jordan) and visualized microscopically for identity assessment. Pre-existing surface-bound IgE antibodies were stripped from FcεRI on basophils following the protocol described elsewhere21 with some modification. Briefly, cells were resuspended in 2 mL of ice cold buffered lactate-salt solution (LS) and incubated for 30 seconds on ice. The cells were neutralized and washed in HE buffer. After that, FcγRs were blocked using a human FcγR blocker (eBioscience Inc. San Diego, CA, USA). Treated cells were tested for surface IgE using FCM, and analysis revealed the absence of surface IgE from LS-treated cells.
Fresh dilutions prepared from 1 mg/mL stock of post- and preimmunized purified camel IgG isotypes at a final volume of 25 µL were incubated with an equal volume of 1:5 diluted atopic human sera for 30 minutes at 37℃. The mixture was added to LS-treated basophils and incubated for 1 hour at 37℃. As a positive control, cells were mixed with 1:5 diluted atopic sera alone. At the end of the incubation period, the cells were washed with HE buffer and labeled with anti-IgE-FITC (Abcam, Cambridge, UK). After incubation for 15 minutes at room temperature in the dark, the cells were washed, and residual erythrocytes were lysed by the addition of 1 mL of erythrocyte lysis buffer (Beckton-Dickinson, Franklin Lakes, NJ, USA). The cells were analyzed by an FCM using FACS Calibur cytometer (Beckton-Dickinson) after gate setting based on forward and side scatter characteristics. The percentage of IgE-binding blocking was calculated by subtracting the % of IgE-labeled cells in the presence of camel IgG treatment from the % of IgE-labeled cells in the absence of camel IgG treatment, and the result was divided by the % of IgE-labeled cells in the absence of camel IgG treatment multiplied by 100.
Fresh dilutions prepared from 1 mg/mL stock of post- and preimmunized purified camel IgG isotypes at a final volume of 25 µL were incubated with an equal volume of 1:5 diluted patient serum for 30 minutes at 37℃. Serum with high IgE titer (concentration: 50 kU/L) obtained from an olive tree-atopic patient attending the Allergy Clinic at Islamic Hospital (Amman, Jordan) was used as the source of huIgE. The mixture was added to LS-treated cells for 1 hour at 37℃. As a positive control, cells were mixed with 1:5 diluted patient serum alone. After washing, the cells were incubated for 1 hour at 37℃ with mouse monoclonal anti-IgE diluted in HE-CM buffer (HE buffer plus 5 mM CaCl2 and 2 mM MgCl2) in a final concentration of 5 µg/mL. To test the ability of this antibody to cross-link with basophil surface-bound IgE, expression of surface CD63 as a cell activation marker was assessed using FCM. Spontaneous histamine release was measured in LS-treated cells incubated with HE-CM buffer alone. At the end of the incubation period, 100 µL of HE-EDTA buffer (HE buffer plus 2.8 mM EDTA) was added, and cell free-supernatant was recovered. Histamine content in the supernatants was analyzed by histamine competition ELISA (IBL-International, Hamburg, Germany) following the specific procedure as per manufacturer instructions.
Fig. 1 shows the kinetics of antibody response in camel immunized with SLP-MAPS. IgG isotypes (IgG1, IgG2, and IgG3) were purified from SLP-MAPS antisera and appeared as pure and clear discrete bands upon SDS-PAGE fractionation (Fig. 2). Evidently, IgG3 appeared as the most dense isotype fraction, while IgG2 was the faintest one. SLP-MAPS-specific isotype analysis showed that the IgG1 isotype was more dominant than IgG3, although only a minute titer of IgG2 response was observed (Fig. 3).
The inhibition potency of IgE binding to its high-affinity receptor (FcεRI) on LS-treated human basophils was measured using purified IgG isotypes prepared from pre- and postimmunized camel sera. Purified IgG2 and IgG3 of postimmunized camel sera showed high blocking activity (72.0% and 96.6%, respectively) when compared to those prepared from preimmunized samples (7.9% and 4.5%, respectively). Lower blocking activity was evident for IgG1 (43.9% for postimmunized and 16.0% for preimmunized) (Fig. 4, Table 1).
The percentage of IHR achieved by camel anti-SLP-MAPS was calculated in the presence of post- and preimmunized camel IgG isotypes. Purified postimmune IgG2 and IgG3 inhibited up to 93.98% and 97.05%, respectively, of histamine release from activated basophils (Table 2). Lower inhibition capability was achieved by conventional IgG1 (60.05%).
This is the first report on the production and functional activity of camel conventional IgG1 and HCAbs (IgG2 and IgG3) prepared against huIgE-Cε3 peptides. The use of a 25-amino acid-long SLP that simulates an active part of Cε3 of the huIgE molecule18 was quite appropriate for the immunization of camels to produce both conventional and HCAbs. Thus, HCAbs (IgG2 and IgG3) produced in camels immunized with SLP designed in the MAPS system proved to be biologically more effective than the conventional IgG1 isotype in interactions with FcεRI on basophils and other cells mediating atopy.
The strategy of peptide immunization for the production of camel anti-huIgE has been adopted following the protocol described previously18 with some modification. In our study, the SLP was conjugated to a core matrix made up of 3 levels of L-lysine for anchoring 8 similar peptide sequences to generate the MAPS system as described earlier.19 In this way, we avoided conjugation to carrier protein and achieved an immune response in camels characterized by the production of both conventional and HCAbs (Figs. 1 and 2). The peptide-specific camel antibodies interacted well with huIgE as proved by ELISA (data not shown) and blocked the interaction of huIgE with its receptors on basophils (Fig. 4). Furthermore, the addition of extra cysteine amino acids achieved cyclization of the peptide by forming intramolecular disulfide bonds.18 This modification increased the degree of conformational resemblance between the peptide and its corresponding region in the cognate intact protein.
In this study, anti-SLP-MAPS-specific IgG2 isotype increase after immunization was lower in comparison to other isotypes. These results are in agreement with those of other studies using synthetic mucin peptides conjugated to BSA as an immunogen.22 Furthermore, this HCAb isotype showed negative ELISA seropositivity using the SLP-MAPS system. This is surprising in light of the fact that it showed a significant blocking potency of IgE on FcεRI present on human basophils. Whether this reflects a higher antibody-binding activity to antigen in solution or superior sensitivity and specificity using the FCM technique remains to be explored. In this regard, Daley et al.23 could not detect IgG2 in the blood of alpacas after natural infection or vaccination with West Nile virus, but IgG2 levels appeared only during the anamnestic response to vaccine. Furthermore, variability in camel isotype response to different immunogens has been documented earlier.222425
In this study, human basophils were stripped from IgE bound to their FcεRI by short incubation in LS buffer for 2 reasons. First, a significant proportion of basophils that express FcεRI were expected to be occupied with endogenous IgE.2126 Second, LS buffer proved to be a safe and suitable one to strip basophils from their IgE bound to FcεRI.2127 Indeed, morphological analysis of cells using microscopic examination as well as FCM showed that the LS-treated cells conserved their cellular integrity and were capable of being resensitized to release histamine efficiently as before treatment. This observation is consistent with others made earlier.21 In fact, FCM showed the presence of IgE-sensitized basophils even after the stripping procedure as indicated by the presence of low fluorescence intensity in the cell population (Fig. 4). However, after cell incubation with atopic sera, a marked shift in fluorescence intensity was attained, indicating that full sensitization has been reached.
Cross-linking of FcεRI on basophils surface with agonists, either allergens or anaphylactogenic anti-IgE antibodies, leads to histamine release. Commercially purchased mouse anti-huIgE was used as an agonist. Its anaphylactogenicity and ability to induce histamine release was tested by FCM through the expression of CD63 activation marker. The significant correlation between the CD63 expression and ability to induce histamine release has been documented.28 Here, mouse anti-huIgE efficiently stimulated basophils activation and CD63 expression as compared to unstimulated basophils (data not shown). Thus, this anti-huIgE was found to be appropriate for use as an agonist. Using both FCM and basophil histamine release, both HCAbs (IgG2 and IgG3) were superior to the conventional camel IgG1 in blocking the IgE FcεRI interaction and IHR. This could be anticipated due to the fact that HCAbs exhibit high affinity in interaction with antigen binding surface due to their extended VHH CDR1 and CDR3 regions that form a convex interactive shape rather than a conical shape formed by the CDRs of VH-VL domains of the conventional IgG1. Moreover, HCAbs have the ability to recognize hidden epitopes due to their small size extending capability and their homodimer nature rather than the hetero-dimer and relatively large size of the conventional IgG1. These characteristics render HCAbs highly efficient for application in toxin neutralization, enzyme inhibition, trypanosome cell lysis, and activation against the tumor necrosis factor and epidermal growth factor receptors as well as the detection of different prostate-specific antigen conformations as reviewed earlier.13 Second, and specifically, IgG3 which is smaller in molecular weight was functionally superior to IgG2 in terms of inhibition of IgE binding to basophils. This observation is consistent with the higher neutralization efficacy of IgG3 against West Nile virus reported earlier.23
In contrast to mouse antibodies, camel HCAbs have low immunogenicity to human due to the high sequence homology between genes comprising their VHH of HCAbs and human VH genes.29 Amino acid sequence analysis revealed that VHH differs from human VH in only 14 amino acids, of which 10 are localized on the surface and the remaining 4 are localized in FR2 of the VHH. This high homology potentiates the humanization process if necessary and renders humanized camel antibody less immunogenic compared to humanized mouse antibody.30 Another factor that favors the use of camel HCAbs and makes them less anaphylactic is the retention of activity while being monovalent.31 In contrast to the bivalent nature of human and mouse MAb, HCAb and its VHH nanobody are active as a single chain or domain format. This supports their feature as non-anaphylactogenic anti-huIgE antibody targeting free serum IgE and limiting their ability to bind to cell-bound IgE. This property potentiates the in vitro production of immunotherapeutic camel HCAbs as there is no need for domain association, and consequently reduces the production cost which will be reflected on the end user price. Such exceptional features of camel HCAbs may overcome some of the adverse effects of omalizumab, the only available approved immunotherapeutic for the treatment of allergy and asthma. Omalizumab is a recombinant humanized mouse monoclonal anti-hu-IgE used for the treatment of most inadequately controlled moderate-to-severe allergic asthmatic patients.323334 Side effects to the use of omalizumab include the ineffectiveness in curing some patients,35 the emergence of malignancies and hypersensitivity reactions, including anaphylaxis, and tolerability problems manifested by injection-site pain, headache, secondary infections, and urticaria.32 Moreover, depending on dosage, the cost of omalizumab may reach as high as $2,706 per month for some patients.36
Numerous unique characteristics of camel HCAbs, such as their high stability and affinity in addition to being functional at high temperature and in the presence of gastric acid and proteolytic enzymes, make them targets for in vivo therapeutic trials. 37383940 Accordingly, HCAbs or their nanobodies will be ideal as orally administered immunotherapeutic agents for the treatment of gastrointestinal and mucosal allergies due to their high proteolytic and gastric-acid stability.
In conclusion, this is the first report on the production, purification, and functional activity of camel polyclonal conventional IgG1 and HCAbs against huIgE-Cε3. IgG2 and IgG3 HCAbs were biologically more active than the conventional IgG1 isotype. Both FCM and IHR tests correlated well in determining camel HCAb's potency to block passive sensitization on human basophils in vitro. Such findings may pave the way toward the possible use of camel anti-huIgE HCAbs as biotherapeutic agents for atopic allergy and asthma using various routes of administration.
Figures and Tables
Fig. 1
Kinetics of antibody response in camel immunized with SLP-MAPS. Camel was immunized 5 times at 2-week intervals with 0.5 mg/dose of SLP-MAPS in combination with Stimune adjuvant at 1:1 ratio.
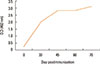
Fig. 2
SDS-PAGE profile of purified camel anti-SLP-MAPS isotypes. Lanes: 1, IgG1; 2, IgG2; 3, IgG3; M, molecular weight markers.
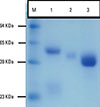
Fig. 3
ELISA of binding activity of purified camel anti-SLP-MAPS isotypes. It shows the O.D of purified anti-SLP-MAPS compared to pre-and postimmunized sera.
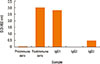
Fig. 4
Flow cytometric analysis of the blocking potency of purified camel anti-SLP-MAPS isotypes to the binding of huIgE to its high affinity receptor (FcεRI) on stripped human basophils. A1-A4 & B1-B4. Fluorescence of anti-IgE-FITC to atopic serum-sensitized human basophils. In A1, atopic serum and camel isotypes are absent. B1 is a complete system in the absence of camel isotypes. B2-B4 Basophils sensitization in the presence of postimmunized camel IgG1 (B2), IgG2 (B3), and IgG3 (B4). A2-A4 Basophils sensitization in the presence of preimmunized camel IgG1 (A2), IgG2 (A3), and IgG3 (A4). Numbers in quadrants represent the percentages of IgE-positive basophils. Blot in the upper left corner of the histogram represent relevant dot blot.

Table 1
Percentage of blocking of IgE binding to human basophils by camel IgG conventional (IgG1) and heavy chain antibodies (IgG2 and IgG3)

Where A1 represents % of IgE labeled cells in the absence of camel IgG treatment (IgE fully occupied receptors, 54.5% as shown in Fig. 4), A2 represents % of IgE labeled cells in the presence of camel IgG treatment.
*The blocking of IgE binding % was calculated as per the formula: ([A1-A2]/A1)*100.
Table 2
Net % inhibition of histamine release (IHR) by prepared human basophils from non-atopic individual sensitized with atopic serum in the presence of purified camel IgG isotypes

ACKNOWLEDGMENTS
This study was supported by the Scientific Research Support Fund (Grant No. 2/22/2008), Jordan.
References
1. Braman SS. The global burden of asthma. Chest. 2006; 130:4S–12S.
2. Myers TR, Tomasio L. Asthma: 2015 and beyond. Respir Care. 2011; 56:1389–1407.
3. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.
4. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008; 8:205–217.
5. D'Amato G. Role of anti-IgE monoclonal antibody (omalizumab) in the treatment of bronchial asthma and allergic respiratory diseases. Eur J Pharmacol. 2006; 533:302–307.
6. Peng Z. Vaccines targeting IgE in the treatment of asthma and allergy. Hum Vaccin. 2009; 5:302–309.
7. Verdoliva A, Rossi M. IgE-mediated disorders: current therapeutics and new strategies involving synthetic peptides. Antiinflamm Antiallergy Agents Med Chem. 2008; 7:252–263.
8. Chang TW. The pharmacological basis of anti-IgE therapy. Nat Biotechnol. 2000; 18:157–162.
9. Hamelmann E. The rationale for treating allergic asthma with anti-IgE. Eur Respir Rev. 2007; 16:61–66.
10. Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993; 363:446–448.
11. Muyldermans S. Single domain camel antibodies: current status. J Biotechnol. 2001; 74:277–302.
12. Muyldermans S, Atarhouch T, Saldanha J, Barbosa JA, Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994; 7:1129–1135.
13. Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, et al. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009; 198:157–174.
14. Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997; 414:521–526.
15. Franco EJ, Sonneson GJ, DeLegge TJ, Hofstetter H, Horn JR, Hofstetter O. Production and characterization of a genetically engineered anti-caffeine camelid antibody and its use in immunoaffinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:177–186.
16. Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 2009; 128:178–183.
17. van de Laa T, Visser C, Holster M, López CG, Kreuning D, Sierkstra L, et al. Increased heterologous protein production by Saccharomyces cerevisiae growing on ethanol as sole carbon source. Biotechnol Bioeng. 2007; 96:483–494.
18. Wang CY, Walfield AM, Fang X, Hammerberg B, Ye J, Li ML, et al. Synthetic IgE peptide vaccine for immunotherapy of allergy. Vaccine. 2003; 21:1580–1590.
19. Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988; 85:5409–5413.
20. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.
21. Pruzansky JJ, Grammer LC, Patterson R, Roberts M. Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J Immunol. 1983; 131:1949–1953.
22. Rahbarizadeh F, Rasaee MJ, Forouzandeh M, Allameh A, Sarrami R, Nasiry H, et al. The production and characterization of novel heavy-chain antibodies against the tandem repeat region of MUC1 mucin. Immunol Invest. 2005; 34:431–452.
23. Daley LP, Kutzler MA, Bennett BW, Smith MC, Glaser AL, Appleton JA. Effector functions of camelid heavy-chain antibodies in immunity to West Nile virus. Clin Vaccine Immunol. 2010; 17:239–246.
24. Conrath KE, Lauwereys M, Galleni M, Matagne A, Frère JM, Kinne J, et al. β-Lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrob Agents Chemother. 2001; 45:2807–2812.
25. Meddeb-Mouelhi F, Bouhaouala-Zahar B, Benlasfar Z, Hammadi M, Mejri T, Moslah M, et al. Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon. 2003; 42:785–791.
26. Helm B, Kebo D, Vercelli D, Glovsky MM, Gould H, Ishizaka K, et al. Blocking of passive sensitization of human mast cells and basophil granulocytes with IgE antibodies by a recombinant human epsilon-chain fragment of 76 amino acids. Proc Natl Acad Sci U S A. 1989; 86:9465–9469.
27. Wan D, Ludolf F, Alanine DG, Stretton O, Ali Ali E, Al-Barwary N, et al. Use of humanised rat basophilic leukaemia cell line RS-ATL8 for the assessment of allergenicity of Schistosoma mansoni proteins. PLoS Negl Trop Dis. 2014; 8:e3124.
28. Sainte-Laudy J, Sabbah A, Vallon C, Guerin JC. Analysis of anti-IgE and allergen induced human basophil activation by flow cytometry. Comparison with histamine release. Inflamm Res. 1998; 47:401–408.
29. Smolarek D, Bertrand O, Czerwinski M. Variable fragments of heavy chain antibodies (VHHs): a new magic bullet molecule of medicine? Postepy Hig Med Dosw (Online). 2012; 66:348–358.
30. Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S, Conrath K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem. 2009; 284:3273–3284.
31. Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007; 77:13–22.
32. Dodig S, Richter D, Cepelak I, Benko B. Anti-IgE therapy with omalizumab in asthma and allergic rhinitis. Acta Pharm. 2005; 55:123–138.
33. Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med. 1999; 341:1966–1973.
34. Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009; 124:1210–1216.
35. Slavin RG, Ferioli C, Tannenbaum SJ, Martin C, Blogg M, Lowe PJ. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009; 123:107–113.e3.
36. Davydov L. Omalizumab (Xolair) for treatment of asthma. Am Fam Physician. 2005; 71:341–342.
37. Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LG, et al. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002; 11:500–515.
38. Harmsen MM, van Solt CB, van Zijderveld-van Bemmel AM, Niewold TA, van Zijderveld FG. Selection and optimization of proteolytically stable llama single-domain antibody fragments for oral immunotherapy. Appl Microbiol Biotechnol. 2006; 72:544–551.
39. van der Linden RH, Frenken LG, de Geus B, Harmsen MM, Ruuls RC, Stok W, et al. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta. 1999; 1431:37–46.
40. Hussack G, Hirama T, Ding W, Mackenzie R, Tanha J. Engineered single-domain antibodies with high protease resistance and thermal stability. PLoS One. 2011; 6:e28218.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download