Abstract
Purpose
Toxoplasma gondii (T. gondii) is an obligate intracellular protozoan parasite that infects humans and animals via congenital or postnatal routes, and it is found worldwide. Modulation of the immune system by parasite infection is proposed to suppress allergic inflammation. Growing evidences have shown that interleukin (IL)-10-producing regulatory B cells (Bregs) and CD4+CD25+FoxP3+ regulatory T cells (Tregs) induced by parasite infection play a critical role in allergic or autoimmune diseases because these cells regulate negatively cellular immune responses and inflammation. Currently, the role of IL-10-producing regulatory B cells in host immune response during T. gondii infection is unknown. In this study, we investigate whether T. gondii infection can suppress the development of unrelated atopic dermatitis (AD)-like lesions.
Methods
AD is a chronically relapsing inflammatory skin disease accompanied by severe itching; for this, we used NC/Nga mice, a well-known experimental model of systemic AD. Repeated exposure to Dermatophagoides farinae crude extract (DfE), known as a major environmental allergen, evokes AD-like skin lesions in NC/Nga mice under specific pathogen-free conditions. NC/Nga mice were intraperitoneally infected with 10 cysts of T. gondii.
Results
T. gondii infection significantly ameliorated AD-like skin lesions in NC/Nga mice. The subpopulation of Bregs and Tregs in the AD mice was expanded in the course of T. gondii infection. In addition, T. gondii infection inhibited Th2 and enhanced Th1 immune response in the DfE-treated AD mice.
Conclusions
We have experimentally demonstrated for the first time that T. gondii infection ameliorated AD-like skin lesions in a mouse model of AD. Our study could in part explain the mechanisms of how parasite infection prevents the development of allergic disorder. Therefore, these immunemechanisms induced by T. gondii infection may be beneficial for the host in terms of reduced risk of allergic immune reactions.
A significant increase in the prevalence of allergic diseasesis becoming an important public health problem in industrialized countries.123 Recent epidemiological studies havesuggested that a steady decline in exposure to viral, bacterial, and parasitic infection is associated with an increase in allergic disorders.45 That is, reduced exposure to microbes and their products in childhood may lead to a failure to development of appropriate immune regulation. Although several experimental studies have consistently shown that parasite infections or parasite-derived products help induction a Th1-biased immunity and prevent the induction of the Th2 system that causes allergic disease,67891011 the mechanism of how parasite infection prevents the development of allergic disorder remains unclear.
Parasites employ various strategies to evade effective host immune response that thwart infection. Although immune evasion has been developed to favor parasite establishment within the host, some particular immune-escaping strategies might, quite paradoxically, be also beneficial for the host.12 Regulatory B cells (Bregs), regulatory T cells (Tregs), and alternatively activated macrophages have been identified as the key components of the immune regulatory network functioning during helminth infections.131415 These immune regulatory cells expand during parasite infection and may prevent the development of unrelated immune-driven pathology, such as allergy and autoimmune diseases.1416
Typically, B cells function as antibody-producing cells, but they are also involved in other immune mechanisms, including cytokine and chemokine secretion and antigen presentation. In addition, B cells have been shown to participate in the induction of immune tolerance and suppression of inflammation, suggesting the existence of IL-10-producing Bregs.161718 The IL-10-producing subset of Bregs was first identified to play a critical role in limiting disease severity in allergic inflammatory and autoimmune conditions.131920 In recent studies, it has also been found that these Bregs are significantly induced during parasite infection.691321 Several studies have demonstrated that in parasite infections, IL-10-producing Bregs are stimulated as part of the parasite-induced host immune response beneficial for the infection.921 Interestingly, IL-10-producing Bregs induced by parasite infection have been shown to suppress allergic inflammatory and autoimmune diseases.91322
Tregs also play essential role in limiting potentially harmful immune-mediated pathology as the negative regulators of the T cell immune response.23 The well-characterized Treg cell type is the CD4+CD25+ T cell expressing the transcription factor FoxP3. Tregs may reduce injurious host inflammatory and immune responses as well as Th2 responses, such as eosinophil activation required to kill parasites.24
Toxoplasma gondii (T. gondii) is a worldwide-distributed intracellular protozoan parasite thatinfectsa variety of warm-blooded mammals and causes one of the most common chronic parasitic infections in humans: approximately one-third of the world population carries T. gondii.25 Toxoplasmosis is usually clinically asymptomatic in healthy individuals but can lead to severe complications in pregnant women and immunocompromised patients.
In animal models, various helminths alleviate the symptoms of experimental allergic and autoimmune diseases via the induction of IL-10-producing Bregs and Tregs.6262728 In addition, a recent study demonstrated that activated Tregs during T. gondii infection contribute to suppression of the development of allergic airway inflammation.29 However, the role of IL-10-producing Bregs induced by T. gondii infection in unrelated immune-driven pathology, such as allergic inflammation, is poorly understood, and there have been no published data on immunomodulation by T. gondii infection in atopic dermatitis (AD) animal models. AD is a chronically relapsing inflammatory skin disease accompanied by severe itching; we used NC/Nga mice, a well-known experimental model of systemic AD, because of the similarity between clinical symptoms displayed in these mice and AD in humans. Models based on NC/Nga mice are thought to provide important information about AD. Repeated exposure to Dermatophagoides farinaecrude extract (DfE), known as a major environmental allergen, evokes AD-like skin lesions in NC/Nga mice under specific pathogen-free conditions.3031
The aim of this study was to examine whether T. gondii infection renders mice less susceptible to AD in a mice model and to investigate the influence of T. gondii infection on the expansion of immune regulatory cells, particularly IL-10-producing CD19+ Bregs and CD4+CD25+FoxP3+ Treg.
Specific pathogen-free 6-week-old female NC/Nga mice were purchased from Japan SLC, Inc. (Tokyo, Japan) and housed in a specific pathogen-free facility in individually ventilated and filtered cages. The animal protocol used in this study was reviewed and approved based on ethical procedures and scientific care by the Korea Centers for Disease Control & Prevention-Institutional Animal care and Use Committee (KCDC-IACUC; Approval Number KCDC-12-039-2A). All animal care and protocols were performed in accordance with the guideline for the Care and Use of Laboratory Animals of Korean Centers for Disease Control.
AD-like skin lesions were induced in 7-week-old female NC/Nga mice using DfE. Ointment containing the components of crude DfE was purchased from Biostir Inc. (Tokyo, Japan). For AD development, the hair on the upper back was shaved, and 200 µL of 4% (w/v) sodium dodecyl sulfate was applied to the shaved dorsal skin and both surfaces of each ear for barrier disruption. After 2 hours, the back skin and both ears of NC/Nga mice were repeatedly treated with 50 mg of ointment containing crude DfE 3 times per week for 4 weeks. Cysts of the T. gondii ME49 strain were obtained from the brains of chronically infected mice. NC/Nga mice were infected with 10 cysts intraperitoneally at 7 days before or after the first application of DfE (Fig. 1A). All mice were sacrificed at the end of the experiment.
For flow cytometry analysis of IL-10-producing CD19+ Bregs, cells in splenocytes were analyzed for the expression of surface markers using the following monoclonal antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD19 (clone 1D3; BD Biosciences, San Jose, CA, USA) and phycoerythrin (PE)-conjugated anti-IL-10 (clone JES5-16E3; eBioscience, San Diego, CA, USA). For subsequent IL-10intracellular staining, the Cytofix/Cytoperm kit (BD Biosciences) was used according to the manufacturer's protocol (BD Biosciences). For the analysis of CD4+CD25+FoxP3+ Treg cells, the mouse Treg-staining kit was used according to the manufacturer's instructions (eBioscience). The stained cells were analyzed by 3-5 color flow cytometry using a FACSversa flow cytometer (BD Biosciences). The FlowJo software (Tree Star Inc., OR, USA) was used to analyze flow cytometry data. To determine background staining, non-reactive iso-type-matched control monoclonal antibodies (eBioscience) were used and gated to exclude ≥98% of non-reactive cells.
The severity of dermatitis was determined macroscopically based on the presence of erythema/hemorrhage, edema, excoriation/erosion, and dryness/scaling and evaluated according to the severity index scoring system. Skin lesions on the back and both ears were scored based on the presence of clinical symptoms: no symptoms=0, mild=1, moderate=2, and severe=3. The total clinical skin severity score was defined as the sum of the individual scores.
Blood samples were collected from the heart, and isolated serum samples were stored at -70℃ until quantitative analysis for Ig subclasses. The concentration of total IgE, IgG1, and IgG2a in mouse serum was measured using the mouse ELISA kit (eBioscience) according to the manufacturer's instructions.
The expression of cytokine, chemokine, and prostaglandin-endoperoxide synthase 2 (COX-2) mRNAin skin lesions was determined by RT-PCR. Total RNA was isolated according to the manufacturer's protocol (Qiagen, Valencia, CA, USA and used to generate cDNA by reverse transcription. PCR was performed with primers specific for each cytokine, chemokine, and COX-2 (Table 1), and cDNA as templates. The amplified products were analyzed by automated capillary electrophoresis (QIAxcel Advance system; Qiagen Irvine, CA, USA) and β-actin expression was used as the internal control.
Dorsal skin from the back and ear lesions was resected, fixed in 10% formalin solution, embedded into paraffin, sectioned, stained with hematoxylin-eosin solution, and examined by light microscopy. Mast cells and eosinophils were stained with toluidine blue and Congo red, respectively. Immunohistochemistry was performed to evaluate the infiltration of CD4+ T cells and dendritic cells into the skin lesions.
We sought to determine whether T. gondii infection can prevent AD-like skin lesions in a mouse model. The onset of skin inflammation as significant hyperplasia of the epidermis and dermis was observed in the DfE-treated mice starting at 2 weeks. The AD-like skin lesions progressively worsened over 4 weeks after the initial treatment. As shown in Fig. 1B, in the control uninfected mice, DfE application initially induced skin dryness, followed by mild erythema, hemorrhage, and edema; finally, the back skin thickened, and severe erythema, hemorrhage, edema, erosion, and excoriation were observed. The dermatitis score (which integrated the individual scores for each symptom) progressively increased starting 2 weeks after the initial DfE application and reached the maximum at 3 weeks (Fig. 1C). Remarkably, NC/Nga mice infected with T. gondii either before or after the development of AD ameliorated AD-like skin lesions (Fig. 1B and C).
Skin-infiltrated eosinophils and mast cells are a characteristic feature of AD.32 The numbers of dermal mast cells and eosinophils markedly increased in the skin lesions of DfE-treated mice (Fig. 2A). In addition, immunohistological staining revealed significant infiltration of CD4+ T lymphocytes and dendritic cells into the dermis (Fig. 2B). In contrast, T. gondii-infected mice showed marked reduction in the thickness of the stratum corneum and in the infiltration of inflammatory cells into the dermis, compared with the results seen for uninfected DfE-treated mice: there were less mast cells and eosinophils in the skin lesions of T. gondii-infected mice (Fig. 2A). Furthermore, we observed that the infiltration of CD4+ T lymphocytes and dendritic cells in the dermis was considerably lower than T. gondii-infected mice in control mice (Fig. 2B).
Skin lesions of patients of AD indicate chronically relapsing inflammatory disorders with prurience and eczema usually associated with elevated serum total IgE levels and a Th2-predominant immune response. It has previously been shown that NC/Nga mice with fully developed skin lesions had markedly elevated serum total IgE levels which correlated with clinical severity of dermatitis.33 Consistent with these results, we observed vigorous production of total IgE and IgG1 in DfE-treated NC/Nga mice (6,065.5±910.7 and 3,419±147.4 µg/mL, respectively) (Fig. 3A). We also examined whether T. gondii infection before or after the development of skin lesions affect serum levels of the Ig subclasses, including IgE, IgG1, and IgG2a. Our results indicate that the generation of IgE and IgG1 in T. gondii-infected NC/Nga mice was clearly inhibited (IgE: 588.4±795.9 and 3,455.4±743.6 ng/mL, P<0.001 and 0.05, respectively; IgG1: 470±369.9 and 759.4±477.9 µg/mL, P<0.001, respectively), whereas IgG2a levels markedly increased (1,943±296.5 and 1,854.1±162.6 µg/mL, respectively) compared to the control group (299.5±131.1 ng/mL, P<0.001) (Fig. 3A).
Next, we examined the mRNA expression of Th1- and Th2-associated cytokines, chemokines, and inflammatory factors in the skin lesions. The mRNA expression of Th2-type cytokines, including IL-4, IL-5, and IL-13, significantly increased in the DfE-treated NC/Nga mice. The infiltration of inflammatory cells into inflammation sites is dependent on the local production of chemokines with leukocyte chemoattractant activity.31 TARC/CCL17 functions as a selective chemoattractant that contributes to local recruitment and migration of Th2 cells expressing CC Tgchemokine receptor 4.3435 The mRNA levels of TARC and the proinflammatory factor COX-2 markedly increased in the skin of DfE-treated mice (Fig. 3B). In contrast, the expression of these Th2-associated inflammatory factors in the skin of DfE-treated mice infected with T. gondii was significantly reduced (Fig. 3B). Furthermore, the parasitic infection led to the elevated expression of Th1-type cytokine IFN-γ and anti-inflammatory cytokine IL-10 in the skin of DfE-treated animals (Fig. 3B).
It has been demonstrated that parasite-induced Bregs or Tregs can suppress Th2-mediated allergic responses.610 To determine whether the expansion of Breg and Treg populations induced by T. gondii infection is relevant to the parasite-mediated suppression of allergic inflammatory response, we assessed the dynamics of these immunoregulatory cells during AD development. As expected, the population of IL-10-producing B cells in spleen was significantly greater in the infected (Tg+ DfE group, 7.11%±2.78%; DfE+ Tg group, 12.03%±3.52%) than in the uninfected DfE-treated NC/Nga mice (2.61%±0.63%, P<0.05 and 0.001, respectively) (Fig. 4A). Although we did not observe the increase in CD4+CD2+FpxP3+Tregs in the mice infected with T. gondii prior to the emergence of skin lesions, the animals infected with the parasite after AD development displayed remarkable increase of Tregs (15.03%±1.4%) compared with the uninfected control mice (8.52%±2.27%, P<0.05) (Fig. 4B).
Recent studies in animal models have demonstrated that chronic helminth infections are strongly associated with a reduced prevalence of inflammatory disorders, including allergic diseases.61327 It has also been shown that parasitic infection may prevent the development of allergic and autoimmune diseases by inducing IL-10-producing B cells and IL-10-dependent mechanisms, suggesting suppressive function of IL-10-producing Breg cells in allergic diseases.1317263637 NC/Nga mice treated with DfE are used as a sensitive animal model for human AD. Most of the population of AD patients has high titers of specific IgE to Dermatophagoides mites.3137 In this study, we examined whether T. gondii infection can inhibit allergic inflammatory response in AD using NC/Nga mice as an experimental AD model. T. gondii infection rendered protection against AD-related pathogenic mechanisms. Histological analysis demonstrated reduced infiltration of inflammatory cells including eosinophils, mast cells, CD4+ T cells, and dendritic cells, into DfE-induced skin lesions of T. gondii-infected NC/Nga mice. Moreover, the high levels of IgE and IgG1 production as well as elevated expression of Th2-associated inflammatory factors observed in AD mice, were significantly decreased in T. gondii-infected AD-like NC/Nga mice. Interestingly, we observed an increase in serum IgG2a and IFN-γ expression in the skin in T. gondii-infected AD-like mice, suggesting that the infection switched the immune response from the Th2 to the Th1 type.
Recent studies have shown that helminthic infections increased IL-10-producing B cells, and that adoptive transfer of these cells from infected mice protected recipient mice against experimentally induced allergic inflammatory disease.613 Indeed, a recent clinical study involving helminth-infected patients with multiple sclerosis also highlighted a role of IL-10-producing B cells in ameliorating disease symptoms.36 Amu et al.6 have demonstrated that Bregs induced by Schistosoma mansoni infection prevent and reverse allergic airway inflammation via Tregs, suggesting that immunoregulatory Bregs and Tregs play an important role in parasite-mediated protection against allergic conditions. Consistent with this notion, our findings reveal that IL-10-producing Bregs and Tregs induced by T. gondii infection can effectively modulate allergic inflammatory responses.
In conclusion, our results demonstrate that T. gondii up-regulates IL-10-producing Bregs and CD4+CD25+FoxP3+ Tregs, which can protect against the development of severe AD. These immune mechanisms induced by T. gondii infection may be beneficial for the host in terms of reduced risk of allergic immune reactions and provide a basis for the development of novel therapeutic strategies to treat chronic AD.
Figures and Tables
Fig. 1
T. gondii infection inhibits atopic dermatitis in NC/Nga mice. (A) Experimental schedule for the development of DfE-induced atopic dermatitis and T. gondii infection in NC/Nga mice. (B) Skin lesions developed by NC/Nga mice treated with DfE. T. gondii was injected 7 days before (Tg+DfE) or after (DfE+Tg) the first DfE application. White and yellow arrows indicate excoriation and desquamation, respectively. (C) The dermatitis score was based on the severity of four symptoms (erythema, scarring, edema, and erosion): 0, no symptoms; 1, mild; 2, intermediate; 3, severe. ***P<0.001 compared with uninfected NC/Nga mice. Graph represent the mean±SD (n=5) of one experiment (three experiments with comparable results were performed). Tg, T. gondii; DfE, Dermatophagoides farinae extract.
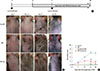
Fig. 2
T. gondii suppressed infiltration of immune cells in the skin of mice with atopic dermatitis. (A) Hematoxylin-eosin staining of the lesioned skin revealed heavy inflammation, hyperkeratosis, acanthosis, and parakeratosis. Infiltration of inflammatory cells into the skin after DfE application was determined by staining tissue sections with toluidine blue or Congo red to detect mast cells and eosinphils, respectively. Original magnification,×200. (B) Immunohistological staining revealed infiltration of CD4+ T cells and dendritic cells in the dermis/epidermis. Paraffin sections were immunostained with anti-CD4 or anti-CD205 antibody to detect CD4-positive cells and dendritic cells, respectively. Positively stained cells are shown in brown color. Arrows indicate dendritic cells in the epidermis. Original magnification,×100. Results shown represent three independent experiment (n=5 for each group). Tg, T. gondii; DfE, Dermatophagoides farinae extract.
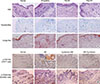
Fig. 3
T. gondii infection changed the immune response from Th2 to Th1 in DfE-induced atopic dermatitis. (A) Blood samples of uninfected DfE-treated Nc/Nga mice (DfE), mice infected with T. gondii 7 days prior to the first DfE application (Tg+DfE), and mice infected with T. gondii 7 days after DfE application (DfE+Tg) were obtained by heart puncture; serum levels of IgE, IgG1, and IgG2a were measured by sandwich ELISA. (B) mRNA expression of Th1- and Th2-associated cytokines, chemokines, and inflammatory factors in the skin lesions. Total RNA was prepared from the back skin and analyzed by RT-PCR. The intensity of PCR bands was measured using the Qiaxcel Advanced System. (C) Transcript levels normalized to β-actin expression used as the internal control. The data are expressed as the mean±SD of three independent experiments (n=5 for each group). *P<0.05 and ***P<0.001 for infected versus uninfected DfE-treated mice. Tg, T. gondii; DfE, Dermatophagoides farinae extract.
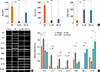
Fig. 4
IL-10-producing Bregs and CD4+CD25+Foxp3+ Tregs subpopulations expand in response to T. gondii infection. The mice were sacrificed at 28 days after first application of DfE.(A) Identification of splenic IL-10-producing CD19+ B cells induced by T. gondii infection in NC/Nga mice with DfE-induced atopic dermatitis. Bar graph indicates the percentage of IL-10+CD19+ B cells. (B) Flow cytometry analysis of CD4+CD25+FoxP3+ T cells population. After staining of splenocytes with CD4 and CD25 antibodies, cells were permeabilized and stained for intracellular FoxP3 using FoxP3 antibody. Bar graph indicates the CD4+CD25+FoxP3+ T cells. *P<<0.05 and ***P<0.001 compared with uninfected DfE-treated NC/Nga mice. The data are expressed as the mean±SD of three independent experiments (n=5 for each group). Tg, T. gondii; DfE, Dermatophagoides farinae extract.

Table 1
Primer sequences for RT-PCR

Notes
References
1. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006; 368:733–743.
2. Ring J, Krämer U, Schäfer T, Behrendt H. Why are allergies increasing? Curr Opin Immunol. 2001; 13:701–708.
3. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001; 1:69–75.
4. Gereda JE, Leung DY, Thatayatikom A, Streib JE, Price MR, Klinnert MD, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000; 355:1680–1683.
5. Garn H, Renz H. Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology. 2007; 212:441–452.
6. Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010; 125:1114–1124.e8.
7. Jeong YI, Kim SH, Ju JW, Cho SH, Lee WJ, Park JW, et al. Clonorchis sinensis-derived total protein attenuates airway inflammation in murine asthma model by inducing regulatory T cells and modulating dendritic cell functions. Biochem Biophys Res Commun. 2011; 407:793–800.
8. Jeong YI, Kim YJ, Ju JW, Hong SH, Lee MR, Cho SH, et al. Identification of anti-allergic effect of Clonorchis sinensis-derived protein venom allergen-like proteins (CsVAL). Biochem Biophys Res Commun. 2014; 445:549–555.
9. Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, et al. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010; 184:886–894.
10. Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005; 202:1199–1212.
11. Kishi C, Amano H, Suzue K, Ishikawa O. Plasmodium berghei infection ameliorates atopic dermatitis-like skin lesions in NC/Nga mice. Allergy. 2014; 69:1412–1419.
12. Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol. 2006; 28:515–523.
13. Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011; 128:733–739.
14. Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012; 33:181–189.
15. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010; 32:593–604.
16. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002; 16:219–230.
17. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002; 3:944–950.
18. Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008; 20:332–338.
19. Lee MK, Lee WY, Yong SJ, Shin KC, Lee SN, Lee SJ, et al. A case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis. Allergy Asthma Immunol Res. 2011; 3:141–144.
20. Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. 2011; 3:168–177.
21. Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. Induction of IL-10-producing CD1d(high)CD5+ regulatory B cells following Babesia microti-infection. PLoS One. 2012; 7:e46553.
22. Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, Filbey K, et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010; 40:1682–1696.
23. Lee JC, Hayman E, Pegram HJ, Santos E, Heller G, Sadelain M, et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 2011; 71:2871–2881.
24. Velavan TP, Ojurongbe O. Regulatory T cells and parasites. J Biomed Biotechnol. 2011; 2011:520940.
25. Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010; 26:190–196.
26. Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004; 173:6346–6356.
27. Smits HH, Hammad H, van Nimwegen M, Soullie T, Willart MA, Lievers E, et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007; 120:932–940.
28. Girgis NM, Gundra UM, Loke P. Immune regulation during helminth infections. PLoS Pathog. 2013; 9:e1003250.
29. Fenoy I, Giovannoni M, Batalla E, Martin V, Frank FM, Piazzon I, et al. Toxoplasma gondii infection blocks the development of allergic airway inflammation in BALB/c mice. Clin Exp Immunol. 2009; 155:275–284.
30. Matsuoka H, Maki N, Yoshida S, Arai M, Wang J, Oikawa Y, et al. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy. 2003; 58:139–145.
31. Oshio T, Sasaki Y, Funakoshi-Tago M, Aizu-Yokota E, Sonoda Y, Matsuoka H, et al. Dermatophagoides farinae extract induces severe atopic dermatitis in NC/Nga mice, which is effectively suppressed by the administration of tacrolimus ointment. Int Immunopharmacol. 2009; 9:403–411.
32. Matsui K, Nishikawa A. Percutaneous application of peptidoglycan from Staphylococcus aureus induces an increase in mast cell numbers in the dermis of mice. Clin Exp Allergy. 2005; 35:382–387.
33. Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997; 9:461–466.
34. Hirata H, Arima M, Cheng G, Honda K, Fukushima F, Yoshida N, et al. Production of TARC and MDC by naive T cells in asthmatic patients. J Clin Immunol. 2003; 23:34–45.
35. Jahnz-Rozyk K, Targowski T, Paluchowska E, Owczarek W, Kucharczyk A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy. 2005; 60:685–688.
36. Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008; 64:187–199.
37. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008; 28:639–650.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download