Abstract
Purpose
Myeloid differentiation-2 (MD-2) has been associated with endotoxin and inflammatory disorders because it can recognize lipopolysaccharide (LPS) binding and attenuate Toll-like receptor 4 (TLR4)-mediated signaling. However, its role in allergic inflammation has yet to be clarified. We examined whether single nucleotide polymorphisms (SNPs) in MD-2 promoter can affect MD-2 expression and aimed to clarify the relationship between Der p 2 allergy and SNPs of MD-2 promoter.
Methods
The function of SNPs of MD-2 promoter and the effects of cytokines and immunoglobulin on the secretion and mRNA expression were investigated in 73 allergic subjects with different MD-2 gene promoter variants. Peripheral blood mononuclear cells were cultured with or without LPS in the presence of Dermatophagoides pteronyssinus group 2 allergen (Der p 2), followed by mRNA extraction and cytokine expression analysis. The culture supernatants were collected for cytokine measurement.
Results
Patients with the MD-2 promoter SNPs (rs1809441/rs1809442) had increased mRNA expressions of MD-2, ε heavy chain of IgE (Cε), and interleukin (IL)-8; however, only MD-2 and IL-8 were further up-regulated after Der p 2 stimulation. Patients with SNPs of MD-2 promoter tended to have high levels of IL-1β, IL-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α after Der p 2 and LPS stimulation. Increased secretions of IL-6, IL-8, and IL-10 were found to be up-regulated by Der p 2 stimulation, and an increased secretion of IFN-γ and decreased secretion of IL-4 were noted after LPS stimulation.
Increasing evidence shows that exposure to indoor allergens is a causative factor for the development of allergic asthma among persons who are genetically predisposed to IgE autoantibody responses.123 Hypersensitivity to house dust mite (HDM) allergens is one of the most common allergic responses, and more than 80% of all patients with allergic asthma are sensitized to HDMs. These patients are characterized by high levels of serum total IgE and allergen-specific IgE to HDMs in Taiwan.456
Dermatophagoides pteronyssinus group 2 allergen (Der p 2) is considered a major allergen of HDM, and 80%-90% of all HDM-allergic subjects have been reported to be sensitive to Der p 2.4 Der p 2 allergens are proteins that tend to bind to the lymphocyte antigen 98 protein, also known as myeloid differentiation-2 (MD-2), which is associated with Toll-like receptor 4 (TLR4) on respiratory epithelial cells.7 MD-2 is an essential component of lipopolysaccharide (LPS) sensing, which upon binding to LPS and TLR4 forms the LPS-MD-2-TLR4 complex that can trigger cellular responses causing inflammatory reactions.78 Der p 2 has structural homology with MD-2, and this allergen has been reported to facilitate signaling through direct interactions with TLR4 in the absence of MD-2.8 It has been reported that mutations of the MD-2 gene on chromosome 8 can affect the human innate response.9 Since our previous reports demonstrated that mite allergen Der p 2 can trigger human B-lymphocyte activation and MD-2/TLR4 induction,10 it is conceivable that Der p2-susceptible genes may be related to the expression of MD-2.
It has been demonstrated that promoter polymorphisms can affect transcription activity and cell surface receptor expression.11 In our previous report, 6 SNPs of MD-2 promoter were identified and differences in genotype and allelic frequency were compared between allergic and healthy subjects; the results showed that the test genotypes of the rs1809441 and rs1809442 were significantly different between the 2 populations (P<0.05).12 MD-2 promoter SNPs (rs1809441/rs1809442) were significantly associated with Der p 2-specific IgE, suggesting that the 2 SNPs may play a major role in susceptibility to Der p 2- triggered immune responses in a Taiwanese population.12 Based on the 1,000 genome project data, the minor allele frequencies of the rs1809441 and rs1809442 are 0.467 and 0.474, respectively. The correlation of pairwise linkage disequilibrium (LD) between the 2 SNPs is D'=1.
The aims of this study were to examine whether promoter polymorphisms in the MD-2 gene can affect MD-2 expression and to clarify the relationship between Der p 2 allergy and SNPs of MD-2 promoter. Functional studies were conducted to investigate the role of MD-2 promoter polymorphisms in the development of allergic inflammation by analyzing the expressions of inflammatory cytokines in peripheral blood mononuclear cells (PBMCs) derived from allergic subjects with different genotypes of MD-2 promoter SNPs.
The Institutional Review Board of Taichung Veterans General Hospital reviewed and approved the ethical issues of this study (IRB Nos. CF12009 and CF12010). Written informed consent was obtained from each participant before they were enrolled in the study. A total of 73 allergic patients who attended the Allergy Clinic at Taichung-Veterans General Hospital were recruited. There were 44 males and 29 females in the study. Their age ranged from 6 to 52 years, with a mean±standard deviation of 34±16. Among the 73 subjects, 45 suffered from allergic rhinitis (nasal symptoms), 38 suffered from atopic dermatitis or urticaria (skin symptoms), and 22 subjects suffered from asthma (airway symptoms); 32 had all the diseases. These subjects had a history of recurrent nasal stuffiness, nasal itching, sneezing, rhinorrhea, and/or asthma as defined according to the allergic rhinitis and its impact on asthma (ARIA) guidelines.1415 Serum total IgE and mite-specific IgE levels were measured using a UniCAP system (ThermoFisher Scientific, Uppsala, Sweden).
Ethylene diamine tetra-acetic acid syringes with 5 mLof blood samples were collected, and DNA from the buffy coat was purified using a Genomic DNA Mini kit (Geneaid, Taoyuan, Taiwan). A set of primers were designed for the amplification of 156 base pair PCR products based on MD-2 promoter. The forward and reverse primer sequences were 5'-TggAgTgTAgTggCCCAATC-3' and 5'-CATggTgAAATTCCgTCTCT-3', respectively. The DNA of the 156 base pair PCR products was cleaned using a PCR DNA Fragments Extraction Kit (Geneaid). The genotypes were determined using a TaqMan real-time PCR (TaqMan SNP Genotyping Assays; Life Technologies, Carlsbad, CA, USA) in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster, CA, USA). Genotyping of 2 SNPs (rs1809441 and rs1809442) I n MD-2 promoter was performed using Custom TaqMan® SNP Genotyping Assay Mix (Rack ID: 4620713-1 and ID: 186819793-1) and TaqMan® Universal PCR Master Mix. The Taqman probe sequences for SNP genotyping of rs1809441 and rs1809442 in MD-2 promoter were AGCCTCCC(G/T)AGTAGC and CCTGGGTT(C/G)AAGCGAT, respectively. Two SNPs were identified at -1265 (rs1809441 G/T) and -1292 (rs1809442 C/G) in MD-2 promoter.
The potential effects of different SNPs on promoter activity were investigated using a luciferase reporter gene assay system. The MD-2 promoter regions from different patterns of genotypes from subjects were constructed in the luciferase reported vector-pGL3 vector (Promega, Madison, Wisconsin, USA). A total of 5 plasmid constructs were prepared, which contained a promoterless (pGL3-basic) or wild genotype of CC (rs1809442)/GG (rs1809441), a single mutant genotype of CG and GT, a combined mutant genotype of GG and TT, or a positive control of SV-40 promoter. The epithelial cell line was transfected with 2 µg of construct plasmids (BEAS-2B) using FuGENE6 reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA). At 24 hours post-transfection, the cells were treated with Der p 2 (10 µg/mL) for 24 hours. Luciferase activities were measured with a Luminoskan Ascent luminometer (Thermo Labsystems, Helsinki, Finland). Results were expressed as the fold increase in relative luciferase activity (RLA) of the MD-2 promoter construct vectors compared to the RLA of pGL3-Basic.
The selection criteria of experiments for human PBMC culture and cytokine analysis were according to the written informed consent and the amount of specimen we obtained from each participant. A total of 73 allergic subjects who attended the allergy and clinical immunology outpatient clinics at Taichung Veterans General Hospital were recruited for the human PBMCs that used for culture. Human PBMCs were prepared by density centrifugation (Ficoll-Paque). The cells were stimulated with or without Der p 2 (10 µg/mL) or LPS (100 ng/mL) for 3 days in RPMI-1640 medium containing 10% heat-inactivated fetal blood serum (FBS) and 1% streptomycin/penicillin in a humidified 5% CO2 atmosphere. Cell culture supernatants were collected and evaluated for cytokine concentrations using commercially available Luminex MAP® kits according to the manufacturer's directions. Correlations between data sets were evaluated using Pearson's correlation coefficient (r).
PBMCs were cultured with or without Der p 2 or LPS (Escherichia coli strain 055:B5; Sigma-Aldrich, St. Louis, MO, USA) for 3 days, followed by RNA extraction. Total RNA from the cells was extracted using an RNeasy mini kit (Qiagen, Hilden, Germany). The first standard cDNA was synthesized by RevertAid M-MuLV reverse transcriptase (Thermo Fisher Scientific, Uppsala, Sweden) according to the manufacturer's protocol. The cDNA then served as a template in a PCR using a G-Storm PCR system. The forward and reverse primers were as follows: MD-2, 5'-AgAAgCAgTATTgggTCTgC-3' and 5'-ggCTCCCAgAAATAgCTTC-3'; GATA-3, 5'-CTCTgCTTCATggATCCCTAC-3' and 5'-CACAgTTCACACACTCCCTg-3'; Cε, 5'-CgTCTTCCCCTTgACCCgCTgCTg-3' and 5'-CACgTCCATgACCTgCCCgTCCTC-3'; Iγ1/2-Cµ, 5'-gggCTTCCAAgCCAACAgggCAggACA-3' and 5'-gTTgCCgTTggggTgCTggAC-3'; IL-1β, 5'-AAACAgATgAAgTgCTCCTTCCAgg-3' and 5'-TggAgAACACCACTTgTTgCTCCA-3'; IL-8, 5'-TTggCAgCCTTCCTgATTTCT-3' and 5'-TCTC AgCCCTCTTCAAAAACTTCTC-3'; and GAPDH, 5'-CCACCCATggCAAATTCCATggCA-3' and 5'-TCTAgACggCAggTCAggTCCACC-3'. The amplification cycles were as follows: 94℃ for 30 seconds, 60℃ for 30 seconds, and 72℃ for 60 seconds. The PCR products were then subjected to electrophoresis on a 2% agarose gel for 30 cycles. The electrophoresis products were visualized by ethidium bromide staining. The mRNA of GAPDH was used to control the sample integrity and loading.
The electrophoretic mobility shift assay was performed according to the manufacturer's instructions as described in a LightShift® Chemiluminescent EMSA kit (Thermo Fisher Scientific Inc. Manufacturer: Pierce Biolechnology, Rockford, Illinois, USA). The oligonucleotide with the tandem GATA motif (-136 to -161) as a probe was 5'-CTCCGTATTTGATAAGGAACAAATAG-3'. The DNA-protein complexes were resolved on a 6% non-denaturing polyacrylamide gel and visualized by exposure to autoradiographic films.
Cytokines in activated helper T (TH) cells derived from the donor's leukocytes underwent immunofluorescence staining and flow cytometry. Three-color staining methods were used to analyze the expression of IL-4 and interferon (IFN)-γ in CD4+ cells. PBMCs from 50-mL peripheral blood of allergic subjects were collected and stimulated with phorbol myristate acetate (PMA; 50 ng/mL), ionomycin (2 µM), and GolgiStop (Cytofix/Cytoperm Plus, Becton Dickinson: BD Pharmingen, Piscataway, NJ, USA) for 5 hours before washing twice with PBS. The cells were stained with peridinin chlorophyll-a protein (PerCP)-conjugated rat anti-human CD4 monoclonal antibody (BD Biosciences, Bedford, MA, USA) at room temperature for 30 minutes and washed with PBS. Cells were fixed with cytofix/cytoperm at room temperature for 30 minutes, washed with PBS, and stained with fluorescein isothiocyanate-conjugated rat anti-human IFN-γ monoclonal antibody (BD Biosciences) and R-phycoerythrin-conjugated rat anti-human IL-4 monoclonal antibody (BD Biosciences) at room temperature for 30 minutes and washed with PBS. The cells were resuspended in 0.5 mL of PBS with 0.1% (wt/vol) sodium azide. Mean fluorescence was measured using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). A total of 5,000 cells were analyzed in each sample.
All statistical analyses were performed using SPSS software (version 22; SPSS Inc, Chicago, IL, USA). SPSS Sample Power 2.0 was used for power calculation analysis. The values are presented as mean±SEM for each group. Non-parametric analysis of the Wilcoxon signed rank and Mann-Whitney U tests were performed to calculate differences between the individual groups. A P value of less than 0.05 was considered statistically significant.
The transcription activity on the MD-2 promoter of SNP No. rs1809441/rs1809442 was measured using a promoter assay. The effect of MD-2 promoter polymorphism transcription activity was analyzed using the transient expression system of a luciferase activity assay without Der p 2 stimulation. The schematic diagram of plasmid construct is shown in Fig. 1A. The promoter activities of several MD-2 promoter genotypes were different, which was significantly higher in MD-2 promoter SNPs (rs1809441-T/rs1809442-G) than in those with the genotype of (rs1809441-G/rs1809442-C) (P<0.01) (Fig. 1B).
The SNPs (rs1809441/rs1809442) of MD-2 promoter were searched for the transcription factor-binding site of Trans-acting T-cell-specific transcription factor-3 (GATA-3), based on the TFMATRIX transcription factor-binding site profile database TFSEARCH version 1.3.16 The sequence fragments of 71 bases were queried with 31 bases of rs1809441/rs1809442. The results showed that sequence fragments of rs1809441 highly correlated with GATA-3, with a score of 91.6. A similarly high correlation score was observed for rs1809442 with GATA-3 (Fig. 1C).
To determine whether the MD-2 promoter SNPs (rs1809441/rs1809442) affect Cε and GATA-3 mRNA expressions, PBMCs derived from allergic subjects were cultured with or without 24, 48, and 72 hours of Der p 2 challenge, followed by mRNA measurement, RT-PCR, and gel analysis presented by representative data (Fig. 2A). When mRNA expressions of Cε and GATA-3 were analyzed, both mRNA expressions could be up-regulated by Der p 2 in both groups of subjects. There were significantly increased expressions of Cε mRNA (Fig. 2B) and GATA-3 (Fig. 2C) with the Der p 2 challenge after 48-72 hours. The expressions of Cε and GATA-3 were significantly higher in allergic subjects with MD-2 promoter SNPs (rs1809441-T/rs1809442-G) compared to MD-2 wild-type (rs1809441-G/rs1809442-C) both in the conditions of PBMC cultured with or without Der p 2 stimulation after 48-72 hours (Fig. 2B and C).
To determine whether the MD-2 promoter SNPs (rs1809441/rs1809442) affect MD-2 mRNA expression, PBMCs derived from allergic subjects were cultured with or without Der p 2, followed by mRNA measurement. There was a significant increase in MD-2 mRNA expression in subjects with MD-2 promoter SNP mutants, and the increase could be further up-regulated by Der p 2 (Fig. 3).
When mRNA and mRNA expressions of Cε and Iγ1/2-Cµ were measured, there was a significantly increased expression of Cε mRNA (Fig. 4A) and a significantly decreased expression of Iγ1/2-Cµ mRNA expression (Fig. 4B). These expressions were not affected by Der p 2 in any of the subjects with or without MD-2 promoter SNPs (rs1809441/rs1809442) (Fig. 4).
When the mRNA expressions of IL-1β and IL-8 were measured, the expression of IL-8 was higher in subjects with MD-2 promoter SNP mutations compared to the wild type, and both IL-1β and IL-8 could be up-regulated by Der p 2 in both groups of subjects. In comparison to the wild type, there were significantly higher expression levels in subjects with MD-2 promoter SNPs (rs1809441/rs1809442) after Der p 2 stimulation, although both IL-1β and IL-8 were up-regulated by Der p 2 (Fig. 5).
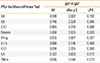
Next, we determined whether MD-2 promoter SNPs (rs 1809441/rs1809442) affect cytokine secretion. A total of 18 subjects were included in this study; 10 of whom had MD-2 promoter SNPs, but 8 did not. PBMCs were cultured with or without Der p 2 and LPS for 3 days, and the supernatants were collected for cytokine measurement using multiplex Enzyme-Linked Immunosorbent assays (ELISA). The results showed that there was a significantly higher level of IL-8 in subjects with MD-2 promoter SNPs (rs1809441/rs1809442) without stimulation. The levels of IL-1β, IL-6, IL-10, and TNF-α were up-regulated after Der p 2 stimulation in subjects with MD-2 promoter SNPs (rs1809441/rs1809442) (Table 1).
When cytokine secretion was compared between subjects with and without MD-2 promoter SNPs (rs1809441/rs1809442), the levels of IL-6, IL-8 and IL-10 were significantly higher in subjects with MD-2 promoter SNPs by Der p 2 stimulation. PBMCs stimulated with LPS showed a significant increase in IFN-γ and decrease in IL-4 in subjects with MD-2 promoter SNP mutations (Table 2).
In order to clarify whether and how MD-2 promoter SNPs affect the binding affinity of GATA-3, and then affect the MD-2 promoter activity, the human promyelocytic leukemia cell line (HL-60) and human monocytes derived from donor' PBMCs (allergic and non-allergic subjects) were employed. The binding activity of GATA-3 to these MD-2 promoter SNPs was analyzed by EMSA using an oligonucleotide containing the tandem GATA motif (-136 to -161). The functional contribution of interactions between GATA-3 and allergen Der p 2 to MD-2 promoter activation, the binding complex was detected with the GATA motif (-136 to -161) probe. The binding activity of transcription factor-GATA-3 induced by Der p 2 (1.5 µg/mL) was in a time-dependent manner (0-8 hours) with the human promyelocytic leukemia cell line HL-60 (Fig. 6A), suggesting that there was a stimulatory effect of Der p 2 on the binding activity of GATA-3 after 4 hours. Furthermore, the monocytes derived from subjects with or without MD-2 promoter SNPs were collected to analyze differences in responses to Der p 2 between different genotypes. There was an obviously enhanced binding affinity of GATA-3 in subjects with MD-2 promoter SNPs (rs1809441-T/rs1809442-G) than in those with the genotype (rs1809441-G/rs1809442-C) after Der p 2 stimulation, suggesting that subjects with MD-2 promoter SNPs display more effective responses to Der p 2 in the binding activity of GATA-3 (Fig. 6B). The results of flow showed that more increased percentages of IL-4+/CD4+ cells after Der p2 stimulation were observed in subjects (patient Nos. 3 and 4) with MD-2 promoter SNPs (rs1809441-T/rs1809442-G) (+) than in those with SNPs (-) (rs1809441-G/rs1809442-C). The percentage of IL-4+/CD4+ cells in subjects (patients Nos. 3 and 4) were 10.65% and 15.95%, respectively, before Der p 2 stimulation, and then 30.00% and 28.99%, respectively, after the Der p 2 stimulation, suggesting that MD-2 promoter SNPs (rs1809441/rs1809442) may affect the binding affinity of GATA-3 and TH2 cytokine expression.
In our previous study, the minor allele frequency of the MD-2 promoter SNPs (rs1809441-T/rs1809442-G) was 0.30 in allergic subjects and 0.17 in healthy subjects. The T allele of rs1809441 and the G allele of rs1809442 were found to be significantly higher in allergic subjects compared to healthy subjects (P<0.05).12 In this study, PBMCs with MD-2 promoter SNPs (rs1809441/rs1809442) were found to have an increased expression of MD-2 and increased secretion of inflammatory cytokines by Der p 2 stimulation. These results indicate that SNPs may be associated with the development of MD-2-related inflammatory diseases. This finding is similar to that of a previous study reporting that SNPs in MD-2 promoter increases MD-2 mRNA production, which correlates with the incidence of sepsis after major trauma.13
Mechanisms underlying the up-regulation of MD-2 expression by Der p 2 cannot be simply due to the structural homology of Der p 2 and MD-2. Der p 2-associated allergic diseases are Th2-dependent, and Th2-cell activation has been shown to be associated with GATA-3 gene expression. Based on the TRANSFAC MATRIX TABLE transcription factor binding site database, sequence fragments of rs1809441 and rs1809442 highly correlate with binding to the transcription site of GATA-3. These results suggest that Der p 2 may trigger MD-2 promoter, followed by activation of GATA-3 and Th2 cells. MD-2 has been reported to play an important role as a cofactor with cell surface TLR-4 and in the recognition of LPS, and group 2 allergenic components of HDM Der p 2 have been reported to serve as an accessory protein in TLR-4 signaling. Therefore, both LPS and Der p 2 may affect host cells synergistically. In our PBMC mRNA expression study, IL-1β and IL-8 were up-regulated by Der p 2 in all subjects, and especially in those with 2 SNP mutants, suggesting that Der p 2 can trigger innate responses in subjects with susceptible MD-2 promoter. The PBMC activation and cytokine (IL-1β and IL-8) release augmented by Der p 2 in these subjects may also have been attributed to the overexpression of MD-2. Similar findings have been reported in which MD-2 expression was up-regulated by Der p 2 in B cells,10 which indicates that the up-regulation of cytokine (IL-1β and IL-8) release may be due to an increased expression of MD-2.
When Cε and Iγ1/2-Cµ mRNA expressions were analyzed, there was no significant augmentation by Der p 2, although the mRNA level of Cε was higher and the level of Iγ1/2-Cµ was lower in subjects with MD-2 promoter SNPs. Therefore, Der p 2-triggered MD-2/TLR4 signaling may only play a partial role in Der p 2-specific IgE synthesis. Other TLR signaling pathways, such as TLR-2 signaling, and other susceptible genes may also be involved in Der p 2-induced B-cell activation and IgE synthesis. According to data from a publicly available database (dbSNP, http://www.ncbi.nlm.nih.gov/SNP), allelic frequencies of genetic variations in the MD-2 or FcεRIα gene promoter region from individuals of different ethnicity are not identical (for example, re1809440 C-allele: 0.479 in African Americans and 0.325 in Europeans). These differences may result in diverse genetic roles of these polymorphisms in different populations with regards to occurrence of certain diseases. Therefore, additional studies are needed to clarify the precise role of these polymorphisms in the pathogenesis of Der p 2 allergy and specific IgE synthesis.
Inconsistent findings on the relationship between exposure to endotoxin and allergic asthma have been reported. It has been hypothesized that LPS can trigger a Th1 response and avoid Th2 prime allergic inflammation. A recent meta-analysis of the association between endotoxin exposure and wheezing/asthma
in children reported a positive association between endotoxin concentration and wheezing in young children, and an inverse association in older children.17
In our cytokine secretion study, PBMCs derived from subjects with MD-2 promoter SNPs (rs1809441/rs1809442) responded differently from those derived from wild type MD-2. Der p 2 could selectively modulate the secretion of the cytokines IL-6, IL-8, and IL-10, and LPS could up-regulate IFN-γ and down-regulate IL-4 secretion, indicating that LPS can prevent Th2-prone allergic responses and that Der p 2 may initiate innate inflammatory responses and contribute to allergic reactions. These findings suggest that both LPS and Der p 2 can trigger inflammatory responses. However, the predetermined genetic background may also play a role in the pathogenesis of allergic inflammation.
It has been reported that the allergenicity of Der p 2 and functional mimicry with MD-2 can facilitate signaling through direct interactions with the TLR4 complex in the absence of MD-2. It has also been reported that allergens from HDMs can induce asthma via TLR4 that triggers airway structural cells to produce innate inflammatory cytokines. This suggests that Der p 2 may activate structural cells directly through its interaction with TLR4 or indirectly through up-regulation of MD-2.1819 In our study, the significantly higher levels of cytokine secretion were found in PBMCs derived from subjects with MD-2 promoter SNPs (rs1809441-T/rs1809442-G) compared to those with the wild type (rs1809441-G/rs1809442-C). Der p 2 could also up-regulate the expression of MD-2. These results suggest that Der p 2 can render PBMCs vulnerable to activation by environmental pathogen endotoxins. It has been reported that the main mite allergen Der p 2 is structurally homologous to MD-2 (also named as LY96 and a molecule required for LPS binding to TLR-4).8 For this reason, Der p 2 induces allergic inflammation in a TLR4-dependent but MD2-independent manner through activation of airway epithelial cells and production of TH2-associated cytokines.8 In addition, the master regulator of transcription factor for TH2-cell differentiation is GATA-3, which promotes TH2 responses through induction of TH2-cytokine production, selective growth of TH2 cells, and inhibition of TH1 cell-specific factors.2021 In this study, the expressions of Cε and GATA-3 in PBMCs derived from allergic subjects were significantly higher in allergic subjects with MD-2 promoter SNPs (rs1809441-T/rs1809442-G) when compared to those with MD-2 wild-type (rs1809441-G/rs1809442-C) both in the conditions of PBMC cultured without and with Der p 2 stimulation. This suggests that MD-2 promoter SNPs may be associated with highly increased expression of the transcription factor GATA-3 and enhanced TH2 cell differentiation.
In conclusion, the results of this study suggest that high secretion levels of proinflammatory cytokines may be predetermined by MD-2 promoter SNPs (rs1809441/rs1809442), which are further up-regulated by Der p 2 and that both genetic and environmental factors can synergistically contribute to allergic inflammation.
Figures and Tables
Fig. 1
Effects of MD-2 promoter SNPs on transcription activity. (A) The promoter activity was investigated using a reporter gene assay system. A total of 5 plasmid constructs were prepared by inserting different genotypes of the MD-2 gene promoter region into a pGL3-Basic vector, which contained a promoterless (pGL3-basic), or genotype of CC (rs1809442) GG (rs1809441), or a single mutant genotype of CG and GT, or a combined mutant genotype of GG and TT, or a positive control of SV-40 promoter. (B) Relative luciferase activity (RLA) was assayed in epithelium cells (BEAS-2B) transfected with different plasmid constructs without Der p 2 stimulation. Luciferase activity was normalized for transfection efficiency using a control plasmid (pGL3-Basic). Results are expressed as the fold increase in RLA of the MD-2 promoter construct vector as compared to pGL3-Basic. The values of luciferase activity are expressed as means±SD of the results from each group (n=5). *P<0.05 compared to the MD-2 promoter genotype of rs1809441-G/rs1809442-C constructs; **P<0.01 compared to the MD-2 promoter genotype of rs1809441-G/rs1809442-C constructs. (C) The scoring scheme was performed by simple routine searches highly correlated sequence fragments versus TFMATRIX transcription factor binding site profile database. Score=100.0* ('weight sum' - min)/( max-min); *, sequence position of SNP rs1809441; #, sequence position of SNP rs1809442.

Fig. 2
The mRNA expressions of ε heavy chain of IgE (Cε) and GATA-3 in PBMCs from subjects with the SNP rs1809441/42 mutant and wild genotypes were evaluated using reverse-transcriptase polymerase chain reaction (RT-PCR). (A) The 0, 24, 48, and 72 represent the time points (hours) after allergen rDer p 2 (10 µg/mL) challenge. The GAPDH expression acted as an internal-control. (B) The Cε expression was evaluated using RT-PCR. Data are expressed as mean of each group. Medium, detected without allergen challenge; Der p 2, detected after rDer p 2 (10 µg/mL) challenge. (C) The expression of the transcription factor GATA-3 was evaluated using RT-PCR; **P<0.05 compared between the MD-2 promoter SNPs (+) and SNPs (-) under medium only; ***P<0.01 compared between MD-2 promoter SNPs (+) and SNPs (-) under Der p 2 (10 µg/mL) challenge. ##P<0.05 compared between medium and rDer p 2 (10 µg/mL) challeges in the group of MD-2 promoter SNPs (+).
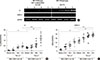
Fig. 3
The SNPs of MD-2 gene promoter affect the MD-2 mRNA expression in PBMC. The MD-2 mRNA expression was evaluated using RT-PCR. Data are expressed as mean of each group. Medium, detected without allergen challenge; Der p 2, detected after the rDer p 2 (10 µg/mL) challenge; *P<0.05 compared between the MD-2 promoter SNPs (+) and SNPs (-) without treatment; **P<0.05 compared between the MD-2 promoter SNPs (+) and SNPs (-) under rDer p 2 challenge; ##P<0.05 compared between medium and rDer p 2 (10 µg/mL) challenge in the group of MD-2 promoter SNPs (+).

Fig. 4
The SNPs of the MD-2 promoter affect Cε and Iγ1/2-Cµ mRNA expressions in PBMCs. The expressions of ε heavy chain of IgE (Cε) and Iγ1/2-Cµ mRNA were evaluated using RT-PCR. Data are expressed as mean of each group. Medium, detected without treatment; Der p 2, detected after the rDer p 2 (10 µg/mL) challenge; LPS, detected after LPS (100 ng/mL) treatment. ##P<0.05 compared between the MD-2 promoter SNPs (+) and SNPs (-) under different treatments.
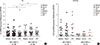
Fig. 5
The SNPs of MD-2 promoter affect the mRNA expressions of IL-1β and IL-8 in PBMCs. The mRNA expressions of IL-1β and IL-8 were evaluated using RT-PCR. Data are expressed as mean of each group. Medium, detected without treatment; Der p 2, detected after the rDer p 2 (10 µg/mL) challenge; ##P<0.05 compared between medium and rDer p 2 challenge; **P<0.05 compared between the MD-2 promoter SNPs (+) and SNPs (-) under medium only; ***P<0.01 compared between MD-2 promoter SNPs (+) and SNPs (-) under Der p 2 (10 µg/mL) challenge.

Fig. 6
MD-2 promoter SNPs (rs1809441/rs1809442) affect the binding affinity of GATA-3 and TH2-cytokine expression. The binding activity of GATA-3 and TH2-cytokine expression were analyzed by electrophoretic mobility shift assay (EMSA) and flow cytometry, respectively. The human promyelocytic leukemia cell line (HL-60) and human monocyte derived from donor' PBMCs were employed for GATA-3 binding activity. The binding activity of GATA-3 to these MD-2 promoter SNPs was analyzed by EMSA using an oligonucleotide containing the tandem GATA motif (-136 to -161). (A) The binding activity of the transcription factor GATA-3 was induced by Der p 2 (1.5 µg/mL) at different time points (0-8 hours) with the human promyelocytic leukemia cell line HL-60. (B) Human monocytes derived from subjects with (+) or without (-) MD-2 promoter SNPs were collected to analyze the binding affinity of GATA-3. SNP (+), MD-2 promoter SNPs (rs1809441-T/rs1809442-G); SNP (-), MD-2 promoter SNPs (rs1809441-G /rs1809442-C). Quantification: hybridization signals were quantified by the image analysis program. (C) Cytokines in activated TH cells derived from donor's leukocytes underwent immunofluorescence staining and flow cytometry. Three-color staining methods were used to analyze the expressions of IL-4 and interferon (IFN)-γ in CD4+ cells. Mean fluorescence was measured using a flow cytometer and is presented in percentages. A total of 5,000 cells were analyzed in each sample.
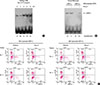
Table 1
Differences in cytokine secretion with and without Der p2 and LPS

Table 2
Comparison between subjects with and without MD-2 promoter SNPs (rs1809441/rs1809442)
ACKNOWLEDGMENTS
This study was supported by the National Science Council, Republic of China (NSC102-2320-B-075A-002) and Taichung General Veterans Hospital, Republic of China (TCVGH-1017302B and TCVGH-1027303B). The authors sincerely appreciate the assistance of the Biostatistics Task Force and the Center for Translational Medicine of Taichung Veterans General Hospital, Taichung, Taiwan.
References
1. Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981; 289:592–593.
2. Boulet LP, Turcotte H, Laprise C, Lavertu C, Bédard PM, Lavoie A, et al. Comparative degree and type of sensitization to common indoor and outdoor allergens in subjects with allergic rhinitis and/or asthma. Clin Exp Allergy. 1997; 27:52–59.
3. Thomas WR, Smith WA, Hales BJ, Mills KL, O'Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002; 129:1–18.
4. Tsai JJ, Shen HD, Chua KY. Purification of group 2 Dermatophagoides pteronyssinus allergen and prevalence of its specific IgE in asthmatics. Int Arch Allergy Immunol. 2000; 121:205–210.
5. Shin JW, Sue JH, Song TW, Kim KW, Kim ES, Sohn MH, et al. Atopy and house dust mite sensitization as risk factors for asthma in children. Yonsei Med J. 2005; 46:629–634.
6. Park SH, Kim ND, Jung JK, Lee CK, Han SB, Kim Y. Myeloid differentiation 2 as a therapeutic target of inflammatory disorders. Pharmacol Ther. 2012; 133:291–298.
7. Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007; 316:1632–1634.
8. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.
9. Vasl J, Prohinar P, Gioannini TL, Weiss JP, Jerala R. Functional activity of MD-2 polymorphic variant is significantly different in soluble and TLR4-bound forms: decreased endotoxin binding by G56R MD-2 and its rescue by TLR4 ectodomain. J Immunol. 2008; 180:6107–6115.
10. Tsai JJ, Liu SH, Yin SC, Yang CN, Hsu HS, Chen WB, et al. Mite allergen Der-p2 triggers human B lymphocyte activation and Toll-like receptor-4 induction. PLoS ONE. 2011; 6:e23249.
11. Hasegawa M, Nishiyama C, Nishiyama M, Akizawa Y, Mitsuishi K, Ito T, et al. A novel -66T/C polymorphism in Fc epsilon RI alpha-chain promoter affecting the transcription activity: possible relationship to allergic diseases. J Immunol. 2003; 171:1927–1933.
12. Liao EC, Chang CY, Wu CC, Wang GJ, Tsai JJ. Association of single nucleotide polymorphisms in the MD-2 gene promoter region with Der p 2 allergy. Allergy Asthma Immunol Res. 2015; 7:249–255.
13. Gu W, Shan YA, Zhou J, Jiang DP, Zhang L, Du DY, et al. Functional significance of gene polymorphisms in the promoter of myeloid differentiation-2. Ann Surg. 2007; 246:151–158.
14. Bousquet J, Van Cauwenberge P, Khaltaev N. Aria Workshop Group. World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001; 108:S147–S334.
15. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.
16. Noguchi T, Matsuda H, Akiyama Y. PDB-REPRDB: a database of representative protein chains from the Protein Data Bank (PDB). Nucleic Acids Res. 2001; 29:219–220.
17. Mendy A, Gasana J, Vieira ER, Forno E, Patel J, Kadam P, et al. Endotoxin exposure and childhood wheeze and asthma: a meta-analysis of observational studies. J Asthma. 2011; 48:685–693.
18. Chiou YL, Lin CY. Der p2 activates airway smooth muscle cells in a TLR2/MyD88-dependent manner to induce an inflammatory response. J Cell Physiol. 2009; 220(2):311–318.
19. Osterlund C, Grönlund H, Polovic N, Sundström S, Gafvelin G, Bucht A. The non-proteolytic house dust mite allergen Der p 2 induce NF-κB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy. 2009; 39:1199–1208.
20. Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006; 16:3–10.
21. Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010; 10:225–235.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download