Abstract
Purpose
Measurement of IgE specific to purified house dust mite (HDM) allergens may improve allergy diagnosis. This study aimed to investigate the sensitization profiles of Korean HDM allergic subjects suffering from respiratory allergy and atopic dermatitis (AD) to Der f 1, Der f 2, Der f 6, Der f 8, Der f 10, and Der f 20.
Methods
Recombinant HDM allergens were produced in Pichia pastoris (Der f 1) or Escherichia coli (5 allergens). IgE reactivity to the individual recombinant allergens and total extract of mite was assessed by ELISA.
Results
Der f 1 was recognized by 79.1%, Der f 2 by 79.1%, Der f 6 by 9.3%, Der f 8 by 6.2%, Der f 10 by 6.2%, and Der f 20 by 6.6% of the patients' sera tested, while the prevalence of IgE reactivity to total mite extract was 94.7%. Combination of Der f 1 and Der f 2 had a sensitivity of 87.6%. Specific IgE to Der f 2 alone was detected from 89.4% of HDM-sensitized respiratory allergy subjects and 92.3% to the combination of the 2 major allergens Der f 1 and Der f 2. However, sera from fewer patients with AD, namely 72.4% and 71.0%, recognized Der f 1 and Der f 2, respectively. The combination of 2 major allergens allowed diagnosis of 84.5% of the AD patients. No correlation between sensitization to specific allergens and HDM allergy entity was found.
Conclusions
Der f 2 was the most frequently sensitized allergen among the HDM-sensitized respiratory and AD patients in Korea, and the combination of the group 1 and 2 major allergens increased the diagnostic sensitivity. Minor allergens did not significantly improve diagnostic sensitivity. However, further studies are needed to analyze the relationship between sensitization to other HDM allergens and the disease entity of the HDM allergy.
The concept of component-resolved diagnosis (CRD) for allergy diagnosis was first introduced in 1999.1 CRD utilizes purified allergens, which are recombinant or native, to identify causative allergens in individual patients. It allows the physician to distinguish genuine sensitization from false positivity by cross-reactivity with other components. It can also discriminate clinically significant sensitization from insignificant sensitization, such as easily denatured allergens in patients with food allergies. Thereby, it enables identification of culprit allergens, can often help select patients who will benefit from allergen-specific immunotherapy,2 and allows appropriate recommendations of what allergens to avoid. In practice, CRD has been found to be useful in diagnosis of food allergies such as peanut, wheat, and buckwheat allergies, in addition to bee sting anaphylaxis or pollen allergy. However, CRD studies of house dust mite allergens are lacking.
A significant correlation between the results of specific IgEs to group 1 or 2 major allergens and the skin prick test has been reported, indicating that CRD might be useful in HDM allergies.3 Variable profiles of IgE reactivity among HDM allergic patients were first reported in Europe.4 A high prevalence of an anti-Der p 4 IgE response, rather than an anti-Der p 1 and Der p 2 response, was described in Australian Aboriginals, indicating distinct allergic responses, possibly in response to different infections.5 Der p 10 (tropomyosin) was suggested as a diagnostic marker of broad sensitization in HDM allergy to discriminate between subjects sensitized exclusively to the major allergens (Der p 1 and Der p 2) and those with a broad sensitization profile.678 Moreover, polysensitization to multiple HDM allergens were correlated with the complexity of the allergic phenotype in children in a tropical environment.9
However, IgE reactivity of some allergens from D. farinae has not been investigated, although the allergenicity of counterpart allergens from D. pteronyssinus has been described. For example, Der f 8 and Der f 20 have not been officially listed as allergens by the allergen nomenclature subcommittee (www.allergen.org) to date.
In this study, we aimed to determine the profile of IgE reactivity in Korean HDM allergic patients and to compare the sensitization profiles of subjects suffering from respiratory allergy and AD using recombinant Der f 1 (cysteine protease), Der f 2 (MD-2 homologue), Der f 6 (chymotrypsin), Der f 8 (glutathione S-transferase), Der f 10 (tropomyosin), and Der f 20 (arginine kinase). Furthermore, we investigated the usefulness of CRD in HDM allergy.
Serum samples were collected from HDM allergy patients in the Allergy-Asthma Clinic at Severance Hospital, Seoul, Korea. Patient consent was obtained before blood collection. Specific IgE to Dermatophagoides farinae was determined using the ImmunoCAP system (Phadia, Uppsala, Sweden). One hundred twenty-nine subjects (62 males and 67 females, mean age 34 years; range, 5 to 79 years) were enrolled in this study. Of 40 subjects with asthma, 15 were diagnosed with asthma (AS) only, 23 with AS and allergic rhinitis (AR), and 2 with AS, AR, and AD. Among 52 patients diagnosed with AR, 27 had AR only, 23 had AR with AS, and 2 had AR, AS, and AD. Of 64 individuals with AD, 58 were diagnosed with AD only and 6 were diagnosed with AD, AR, and AS. Four of these patients were also diagnosed with allergic conjunctivitis (AC). However, statistical analysis was not done for patients with AC due to the small size of this group. Subjects were assigned to the respiratory allergy group, AD group, or both allergy groups for further analysis (Fig. 1). This study was approved by the institutional review board of our institute (4-2013-0397).
Allergen extract was prepared as previously described.1011 Briefly, allergens were extracted with bicarbonate buffer from HDM bodies which were purified using saturated salt water. The extract was extensively dialyzed against distilled water and lyophilized. For the experiments, the extract was reconstituted in a phosphate buffer, aliquoted, and kept at -70℃ until use. Protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA, USA).
Recombinant Der f 1 (proDer f 1) was produced as described by Yasuhara et al. with slight modification.12 Reverse transcriptase (RT)-PCR was carried out using oligonucleotide primers (forward: 5'-CTCGAGCGTCCAGCTTCAATCAAAACT-3', reverse: 5'-GCGGCCGCTTAGTGATGGTGATGGTGATGCGCGCCGCGTGATGGTG-3') to amplify Der f 1.0101, which is the predominant isoform in Korea.13 The underlined sequences corresponded to Xho I and Not I sites, and codons for 6 histidines were also incorporated in the reverse primer. PCR products were ligated into the pCR4-TOPO vector (Invitrogen Life Technologies, Carlsbad, CA, USA). To avoid hyperglycosylation, the 53rd amino acid (N-glycosylation site) was mutated from Asp to Gln using the QuikChange®II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). The mutated plasmid was subcloned into the Xho I and Not I sites of the pPIC9 vector (Invitrogen). Subsequently, Sal I-linearized plasmid was transformed into GS115 cells using the PichiaEasyComp kit (Invitrogen). His+ transformants were selected on RDB plates (1.34% yeast nitrogen base without amino acids, 1 M sorbitol, 1% dextrose, 4×10-5% biotin, and 0.005% each of L-glutamic acid, L-methionine, L-lysine, L-leucine, and L-isoleucine). A clone was selected, and cells were grown for 4 days at 220 rpm; 0.05% methanol (v/v) was added every 24 hours. Culture supernatant was harvested by centrifugation and concentrated by ammonium sulfate precipitation (50%). Precipitates were dissolved in 10 mM imidazole, 300 mM NaCl, and 50 mM sodium phosphate at pH 8.0. Recombinant proteins were purified using Ni-nitrilotriacetic (NTA) acid resin (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Mature Der f 1 was obtained by an auto-activation (cleavage of prosequence) process by dialysis against 100 mM acetate buffer (pH 4.0) at 4℃ for 48 hour.12 After this activation process, recombinant Der f 1 protein was dialyzed against 20 mM Tris-HCl at pH 8.0.
Der f 2.0102 was produced because it is the predominant isoform in Korea.13 DNA fragments were amplified by PCR (forward primer: 5'-GATCAAGTCGATGTTAAAG-3', reverse: 5'-TTATCACGGATTTTACCATG-3') using a clone ligated into a plasmid vector (pCR4-TOPO) (Invitrogen) as template, and these fragments were then subcloned into the pEXP-5NT/TOPO vector (Invitrogen). Der f 6 was amplified by RT-PCR with specific primers (forward: 5'-GATGCACGATTTCCACGC-3' reverse: 5'-TCAAACAATGTTTTTTGT-3'). PCR-products were cloned into the pEXP-5NT/TOPO vector. Der f 8 was also amplified by RT-PCR with primers (forward: 5'-ATGGCTCCAAAAACAATTCTT-3', reverse: 5'-TTATGCATATGTACCATTCCA-3'), and then cloned into the pEXP-5NT/TOPO vector. Der f 10 was PCR-amplified from the pGEM-T Easy vector (Promega) and subcloned into the pET28b expression vector (Novagen, Madison, WI, USA) as previously described.14
For production of recombinant Der f 20, PCR was performed with specific primers (forward primer: 5'-CCATGGTTGATCAAGCTGTCATCG-3', reverse primer: 5'-CTCGAGCATGGATTTTTCAATTTTAAT C-3') designed on the basis of the known cDNA sequence of arginine kinase from Dermatophagoides farinae in GenBank (accession no. AY211951). Underlined sequences correspond to Nco I and Xho I sites, respectively. Amplified PCR product was ligated into the expression vector pET28b (Novagen) after digestion with Nco I and Xho I restriction endonucleases.
All recombinant allergens except Der f 1 were expressed in Escherichia coli BL21 (DE3) and purified using Ni-agarose resin (Qiagen). Der f 2, Der f 6, and Der f 20 were isolated from inclusion bodies using 6 M urea. Der f 8 and Der f 10 were purified from soluble fractions. The concentrations of purified proteins were determined by Bradford assay, and a 10-µg aliquot of each protein was run on a 15% polyacrylamide gel containing sodium dodecyl sulfate under reducing conditions.
Levels of allergen-specific serum IgEs were measured by ELISA. Each allergen (2 µg/mL for recombinant protein and 10 µg/mL for allergen extract) in 50 mM carbonate buffer (pH 9.6) was coated onto a microtiter plate and kept at 4℃ overnight. After blocking with 3% skim milk in phosphate-buffered saline containing 0.05% Tween 20 (PBST), plates were incubated for 1 hour with serum samples at 1:4 dilution. Subsequently, plates were incubated with biotinylated goat anti-human IgE at 1:1,000 dilution (Vector, Burlingame, CA, USA) for 1 hour and streptavidin-peroxidase (Sigma-Aldrich, Sydney, Australia) at 1:1,000 dilution for the detection of IgE antibodies. Plates were washed three times with PBST between each step. Color was developed using 3,3'5,5'-tetramethylbenzidine substrate solution (Kirkegaard Perry Laboratories, Gaithersburg, MD, USA), and then absorbance at 450 nm was measured. The mean plus 2 SD of absorbance values of sera from non-sensitized subjects was used as the cutoff value.
Multivariate regression analysis was performed to analyze statistical significance. Correlations of IgE antibody responses between groups of patients were assessed by Pearson's χ2 test and Fisher's exact test. Correlations between the numbers of subjects diagnosed with allergies according to allergy type, and the number of sensitized allergens were analyzed by ANOVA with Bonferroni correction. SPSS v18.0 was utilized, and a result was considered statistically significant when the P value was less than 0.05.
All expressed recombinant allergens showed an apparent single band with the expected molecular weight on SDS-PAGE analysis. However, Der f 2 appeared as a strong dimer along with a monomer (Fig. 2).
Subjects suffering from respiratory allergies (AR, AS, and/or AC) and/or from AD were analyzed for IgE reactivity to recombinant Der f 1, Der f 2, Der f 6, Der f 8, Der f 10, and Der f 20 by ELISA (Fig. 3). As expected, most subjects had IgE responses to group 1 and 2 allergens. Der f 1- and Der f 2-specific IgEs were found in 86.4% and 89.4% of respiratory allergic patients (n = 65), respectively. Only 4.6 to 6.2% of HDM respiratory allergy patients had specific IgE to each minor allergen (Der f 6, Der f 8, Der f 10, and Der f 20). Anti-group 1 and 2 IgEs were found in 72.4% and 71.0% of AD patients (n=58), respectively. Only 5.2 to 12.1% of the AD patients had specific IgEs to these minor allergens. When all HDM allergy patients were considered, 79.1% and 79.1% of patients had IgEs specific to both group 1 and 2 major allergens, respectively, while only 6.2% to 9.3% of subjects had IgE specific to each minor allergen.
Diagnostic sensitivity according to combinations of various recombinant allergens was determined (Table 1). Der f 2 showed the highest diagnostic sensitivity in respiratory allergic patients (89.4%). The combination of 2 major allergens increased the sensitivity to 92.3%. The combination of all minor allergens with major allergens only resulted in the diagnosis of one additional subject. Approximately 84.5% of AD patients could be diagnosed as HDM allergy by the combination of 2 major allergens. Minor allergens did not increase sensitivity. In patients with both HDM-sensitized respiratory allergy and AD, neither Der f 1 (66.7%) nor Der f 2 (50.0%) was useful for diagnosis.
To investigate the usefulness of CRD for the differentiation of each HDM-related allergic disease, the relationship between sensitization profile and disease entity were examined statistically (Table 2). HDM-sensitized respiratory allergic patients had Der f 2-specific IgE more frequently than AD patients (P=0.013). The mean number of sensitized allergens tested in all enrolled subjects was 1.85, and it was not different between the HDM respiratory allergy and AD groups. There was no statistically significant relationship between the number of sensitized allergens per patient and the number of disease entities such as AS, AR, AC, or AD.
HDM is one of the most frequent causes of respiratory allergy and AD, and 24 groups of allergens have been described to date.15 We investigated patient sensitization profiles to recombinant HDM allergens. Der f 2 allowed diagnosis of the vast majority of respiratory allergic patients (89.4%), while the addition of Der f 1 increased the sensitivity slightly (92.3%). However, the combination of these 2 allergens was less efficient for the diagnosis of AD subjects. These subjects had a sensitivity of 72.4% and 71.0% to Der f 1 and Der f 2, respectively. The combination of two major allergens enabled diagnosis of 84.5% of AD patients. We did not analyze patients suffering from both entities because only 6 of these patients were enrolled. Fifteen subjects did not show a positive reaction to any of the allergens tested. Specific IgE to D. farinae (d2) was low (0.89 to 15.6 kU/L) in these 15 patients, except for 1 serum sample (56 kU/L) as measured by ImmunoCAP. Therefore, sensitivity of ELISA in this study may influence the diagnostic sensitivity using recombinant allergens. The lower IgE reactivity to Der f 1 (62/103, 60.2%) and Der f 2 (63/103, 61.2%) reported in a previous study may be due to the quality of allergens produced.16
We examined whether sensitization to a specific allergen is disease-specific or not. No correlation was found between the sensitization to specific HDM allergens and the disease entity; there was also no relationship between the number of sensitized allergens per patient and the number of disease entities per patient (Table 2). Therefore, the hypothesis that the more allergens a subject is sensitized, the more likely that a subject suffers from multiple disease entities is questionable.9
Among 25 patients with sensitization to minor allergens, only 1 subject (4.0%) showed negative responses to the 2 major allergens. Among 9 subjects that showed IgE reactivity to Der f 10, 7 (77.8%) exhibited positive responses to the other minor allergens. However, 16 subjects were sensitive to the other minor allergens without reactivity to Der f 10. This result implies that Der f 10 is not suitable for use as a broad sensitization marker. Minor allergens analyzed in this study are known to be highly cross-reactive, and sensitization to Der f 10 may imply a history of infection to parasites or cross-reactions with other allergens. Der f 10, tropomyosin, is a well-known invertebrate pan-allergen.17 Infection with intestinal parasites, such as Ascaris lumbricoides, has been reported to be associated with IgE reactivity to tropomyosin.1819 Cross-reactivity of arginine kinase (Der f 20) has also been described.20 This is the first study to report the allergenicity of Der f 20. Several lines of evidence indicate that Der f 8, glutathione S-transferase, is a cross-reactive allergen between parasites and mites, and even cockroaches.2122
Der f 4, Der f 5, Der f 7, and Der f 21 are known as mid-range allergens that bind IgE in 30% to 50% of mite allergic patients.2324 However, these allergens were not included in this study. Further analysis with these mid-range allergens is necessary to investigate the usefulness of CRD in house dust mite allergy.
In conclusion, Der f 2 had diagnostic value in respiratory allergic patients. The combination of Der f 1 and Der f 2 allowed diagnosis of 92.3% of respiratory allergic patients. Of patients with AD, 71.0% had specific IgE to Der f 2, and sensitivity increased to 84.5% when sensitivity to Der f 1 was included. These data imply that careful selection of patient groups could improve diagnostic sensitivity using recombinant allergens. However, we did not find any association between sensitization to a specific allergen and specific disease entity. Furthermore, Der f 10 was not useful as a marker for broad sensitization to minor allergens. Future studies should be done for assessing the usefulness of CRD in HDM allergy using the other recombinant HDM allergens not tested in this study.
Figures and Tables
 | Fig. 1Patient groups with HDM allergies. Respiratory allergy group consisted of subjects diagnosed with asthma (AS), allergic rhinitis (AR), and/or allergic conjunctivitis (AC), while the skin allergy group consisted of those diagnosed with atopic dermatitis (AD). |
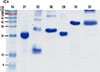 | Fig. 2Production of recombinant HDM allergens. Purified each allergen (10 µg) were separated on a 15% polyacrylamide gel containing sodium dodecyl sulfate under reducing conditions. M, molecular mass standard; 01, recombinant Der f 1; 02, recombinant Der f 2; 06, recombinant Der f 6; 08, recombinant Der f 8; 10, recombinant Der f 10; 20, recombinant Der f 20. |
 | Fig. 3IgE reactivities of recombinant allergens. Absorbance range (A) and positive rate (B) to each allergen are plotted. The bottom and top edges of the box indicate the intra-quartile range (25th and 75th percentiles, respectively). The horizontal line inside the box indicates the median value. Whiskers denote the 10th and 90th percentiles. |
Table 1
IgE binding frequencies to each allergen

Table 2
Serum IgE responses to mite allergens and clinical diagnosis

ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Healthcare Technologies R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI13C0010). This work was also supported by the Brain Korea 21 PLUS Project for Medical Science, Yonsei University.
References
1. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exp Allergy. 1999; 29:896–904.
2. Treudler R, Simon JC. Overview of component resolved diagnostics. Curr Allergy Asthma Rep. 2013; 13:110–117.
3. Taketomi EA, Silva DA, Sopelete MC, Gervásio AM, Alves R, Sung SJ. Differential IgE reactivity to Der p 1 and Der p 2 allergens of Dermatophagoides pteronyssinus in mite-sensitized patients. J Investig Allergol Clin Immunol. 2006; 16:104–109.
4. Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, et al. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008; 38:959–965.
5. Hales BJ, Laing IA, Pearce LJ, Hazell LA, Mills KL, Chua KY, et al. Distinctive immunoglobulin E anti-house dust allergen-binding specificities in a tropical Australian Aboriginal community. Clin Exp Allergy. 2007; 37:1357–1363.
6. Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, et al. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004; 34:597–603.
7. Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, et al. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy. 2011; 41:1468–1477.
8. Mohamad Yadzir ZH, Misnan R, Abdullah N, Bakhtiar F, Leecyous B, Murad S. Component-resolved diagnosis (CRD): Is it worth it? Frequency and differentiation in rhinitis patients with mite reactivity. Iran J Allergy Asthma Immunol. 2014; 13:240–246.
9. Kidon MI, Chiang WC, Liew WK, Ong TC, Tiong YS, Wong KN, et al. Mite component-specific IgE repertoire and phenotypes of allergic disease in childhood: the tropical perspective. Pediatr Allergy Immunol. 2011; 22:202–210.
10. Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, Lee JS, et al. Standardization of house dust mite extracts in Korea. Allergy Asthma Immunol Res. 2012; 4:346–350.
11. Jeong KY, Lee JH, Kim EJ, Lee JS, Cho SH, Hong SJ, et al. Current status of standardization of inhalant allergen extracts in Korea. Allergy Asthma Immunol Res. 2014; 6:196–200.
12. Yasuhara T, Takai T, Yuuki T, Okudaira H, Okumura Y. Biologically active recombinant forms of a major house dust mite group 1 allergen Der f 1 with full activities of both cysteine protease and IgE binding. Clin Exp Allergy. 2001; 31:116–124.
13. Jeong KY, Lee IY, Yong TS, Lee JH, Kim EJ, Lee JS, et al. Sequence polymorphisms of Der f 1, Der p 1, Der f 2 and Der p 2 from Korean house dust mite isolates. Exp Appl Acarol. 2012; 58:35–42.
14. Jeong KY, Lee H, Lee JS, Lee J, Lee IY, Ree HI, et al. Molecular cloning and the allergenic characterization of tropomyosin from Tyrophagus putrescentiae. Protein Pept Lett. 2007; 14:431–436.
15. Jeong KY, Park JW, Hong CS. House dust mite allergy in Korea: the most important inhalant allergen in current and future. Allergy Asthma Immunol Res. 2012; 4:313–325.
16. Park JW, Nahm DH, Hong CS. Determination of specific IgE to two major allergens (Der f I and Der f II) of house dust mite (D. farinae) in Korean adult respiratory allergy patients. Allergy. 1993; 13:476–486.
17. Jeong KY, Hong CS, Yong TS. Allergenic tropomyosins and their cross-reactivities. Protein Pept Lett. 2006; 13:835–845.
18. Arruda LK, Santos AB. Immunologic responses to common antigens in helminthic infections and allergic disease. Curr Opin Allergy Clin Immunol. 2005; 5:399–402.
19. Santos AB, Rocha GM, Oliver C, Ferriani VP, Lima RC, Palma MS, et al. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. 2008; 121:1040–1046.e1.
20. Binder M, Mahler V, Hayek B, Sperr WR, Schöller M, Prozell S, et al. Molecular and immunological characterization of arginine kinase from the Indianmeal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J Immunol. 2001; 167:5470–5477.
21. Santiago HC, LeeVan E, Bennuru S, Ribeiro-Gomes F, Mueller E, Wilson M, et al. Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J Allergy Clin Immunol. 2012; 130:248–256.e9.
22. Acevedo N, Mohr J, Zakzuk J, Samonig M, Briza P, Erler A, et al. Proteomic and immunochemical characterization of glutathione transferase as a new allergen of the nematode Ascaris lumbricoides. PLoS One. 2013; 8:e78353.
23. Thomas WR, Heinrich TK, Smith WA, Hales BJ. Pyroglyphid house dust mite allergens. Protein Pept Lett. 2007; 14:943–953.
24. Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013; 190:3059–3067.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download