Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
Fig. 1
Proportion of Toxocara grade according to variables in the study population. (A) The proportion of Toxocara grade according to history of raw-food intake. (B) The proportion of Toxocara grade according to the amount of raw liver intake. (C) The proportion of Toxocara grade according to peripheral blood eosinophilia and serum hyperIgEaemia. Paticipants were divided into 3 groups according to Toxocara grade: those with Toxocara optical density (OD) less than a weak-positive control OD (negative group), those with Toxocara OD between weak positive and strong positive control ODs (weak positive group), and those with Toxocara OD greater than the strong-positive control OD (strong positive group).
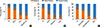
Fig. 2
Standardized Toxocara values according to variables in the study population. (A) The median standardized Toxocara values (interquartile range) were 0.26 (0.06-0.61) in the Toxocara-seronegative group and 2.38 (1.66-2.93) in the Toxocara-seropositive group. (B) The median standardized Toxocara values were 0.36 (0.07-0.95) in participants without history of raw food intake, 0.78 (0.15-1.93) in those with history of raw meat intake, 1.96 (0.96-3.10) in those with history of raw liver intake, and 2.29 (1.33-2.88) in those with history of both raw liver and raw meat intake. (C) The median standardized Toxocara values were 0.49 (0.09-1.27) in participants with no raw liver intake, 1.64 (0.77-2.51) in those with a small amount of raw liver intake, 2.53 (1.80-2.93) in those with a moderate amount of raw liver intake, and 2.62 (1.71-3.36) in those with a large amount of raw liver intake, (D) The median standardized Toxocara values were 0.67 (0.16-1.57) in participants with normal eosinophil count and IgE level, 0.85 (0.28-2.53) in those with peripheral blood eosinophilia, 2.14 (1.36-2.86) in those with serum hyperIgEaemia, and 3.12 (2.63-3.28) in those with both peripheral blood eosinophilia and serum hyperIgEaemia.

Table 1
Demographic characteristics of the study population (N=633)

Variables are expressed as number (percentages), or median (interquartile range).
*Seoul, Incheon, Daejeon, Daegu, Busan, Ulsan, and Gwangju.
A serologic diagnosis of Toxocara infection was done by measuring the specific IgG antibody to T. canis with a Toxocara ELISA kit (Bordier Affinity Products, Crissier, Switzerland). Participants were divided into 3 groups according to Toxocara grade: those who had Toxocara OD less than that of the weak positive control OD (negative group), those who had Toxocara OD between the weak and strong positive control ODs (weak positive group), and those who had Toxocara OD greater than the strong positive control OD (strong positive group).
Table 2
Clinical characteristics of the study population (N=633)

Variables are expressed as number (percentages), or median (interquartile range). Toxocara grade as in Table 1.
OD, optical density.
Table 3A
Crude prevalence ratios of Toxocara seropositivity according to associated factors

Table 3B
Adjusted prevalence ratios Toxocara seropositivity according to associated factors

Table 4A
Crude odds ratios for Toxocara grade according to variables suggesting active infection

Values are odds ratio (95% confidence interval). Estimated from multinominal logistic regression model that used Toxocara grade as outcomes categorized as negative, weak-positive, and strong-positive. Reference category is the negative group. *P<0.05, †P<0.01, ‡P<0.001 (two-tailed tests).
OD, optical density.
Table 4B
Adjusted odds ratios for Toxocara grade according to variables suggesting active infection

Values are odds ratio (95% confidence interval). Estimated from multinominal logistic regression models that used Toxocara grade as outcomes categorized as negative, weak positive, and strong positive. §The model was adjusted for age, sex, residence, and history of raw food intake. Reference category is the negative group. *P<0.05, †P<0.01, ‡P<0.001 (two-tailed tests).
OD, optical density.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download