Abstract
Purpose
Chronic rhinosinusitis (CRS) is characterized by the excessive production of mucus. However, the molecular mechanism underlying mucin overproduction in CRS with or without nasal polyps (CRSwNP and CRSsNP, respectively) is poorly understood. This study was conducted to assess the importance of the transcription factor FoxA2 in mucin production and to investigate the targeting of FoxA2 as a potential therapeutic strategy for mucus hypersecretion in CRS patients.
Methods
We enrolled 15 CRSwNP patients, 15 CRSsNP patients, and 10 normal controls in this study. The expression levels of FoxA2, MUC5AC, and MUC5B in inflamed and healthy nasal tissues were examined via immunohistochemistry and quantitative reverse transcription-polymerase chain reaction, and the levels of several proinflammatory cytokines in nasal secretions were measured via FlowCytomix analysis. In addition, the expression of MUC5AC and FoxA2 was determined in polyp-derived epithelial cells and NCI-H292 cells after in vitro stimulation.
Results
FoxA2 was significantly down-regulated, and MUC5AC and MUC5B were significantly up-regulated in both the CRSwNP and CRSsNP patients compared to the controls (P<0.05), and the protein level of FoxA2 was negatively associated with the IL-6 level in the CRS patients (P<0.05). IL-6 significantly increased MUC5AC expression but inhibited FoxA2 expression in vitro (P<0.05). Transfection with a FoxA2 expression plasmid significantly decreased MUC5AC promoter activity (P<0.05) and inhibited IL-6-induced MUC5AC production (P<0.05). In addition, clarithromycin significantly alleviated IL-6-induced FoxA2 suppression and decreased MUC5AC expression in vitro (P<0.05).
Chronic rhinosinusitis (CRS) is a common disease that affects 4%-10% of the global population. CRS is generally classified into 2 subtypes: chronic rhinosinusitis without nasal polyps (CRSsNP) and chronic rhinosinusitis with nasal polyps (CRSwNP).1 Goblet cell hyperplasia and increased mucin expression are important pathological characteristics of both CRSsNP and CRSwNP, and they account for common symptoms, such as rhinorrhea and nasal congestion.2 Several mucins, including MUC2, MUC4, MUC5AC, and MUC5B, are expressed in the human airways. Of these mucins, MUC5AC and MUC5B are the predominant components of airway mucus. High-density mucin glycoproteins greatly determine the elasticity and viscosity of airway mucus. Although various studies have investigated mucin hypersecretion in CRS,34 molecular mechanisms underlying mucus hypersecretion and the overexpression of MUC5AC and MUC5B have yet to be completely understood.
Recently, the winged helix factor FoxA2 has been linked to increased mucus production via its modulation of mucin gene expression in the airway epithelium. FoxA2 is an essential member of the transcription factor network that represents a common signaling node for multiple goblet cell differentiation signaling pathways.5 The deletion of FoxA2 from mouse lung epithelial cells has been demonstrated to result in goblet cell hyperplasia, and the down-regulation of FoxA2 is associated with increased numbers of MUC5AC-positive goblet cells in transgenic mice.6 To date, the expression of FoxA2 and its potential modulation of excessive mucin production in CRS remain unclear.
IL-6 is a pleiotropic cytokine that exerts beneficial effects on innate immunity and Th17 differentiation.78 IL-6 expression is increased in both subtypes of CRS.910 Chen et al.11 demonstrated that IL-6 and IL-17A, but not Th2-related cytokines, directly stimulate MUC5AC and MUC5B gene expression in human tracheobronchial epithelial cells. Previously, we reported that IL-6 levels are elevated in the nasal tissue of CRS patients, which is associated with enhanced NF-κB activity and regulatory T-cell deficiency.1213 To investigate whether IL-6 promotes mucin production by modulating FoxA2 expression, we examined the mRNA and protein expression levels of FoxA2, MUC5AC, and MUC5B in CRSsNP and CRSwNP patients and evaluated the regulatory effect of IL-6 on FoxA2 expression and the effect of macrolide (clarithromycin [CAM]) on IL-6-induced FoxA2 suppression in vitro.
Thirty CRS patients (15 CRSwNP and 15 CRSsNP patients) were included in this study. The diagnoses of CRSwNP and CRSsNP in this study were based on the patient's clinical history and anterior rhinoscopy/nasal endoscopy/computed tomography (CT) results in accordance with the EPOS guidelines.1 Atopic status was evaluated using skin prick tests for common inhalant allergens. All medications (steroids, antihistamines, and antibiotics) were withheld for a minimum of 4 weeks before the study. The polyp tissues from the CRSwNP patients and the uncinate processes from the CRSsNP patients were sampled under local anesthesia during endoscopic inspection. As controls, 10 patients undergoing transnasal optic nerve decompression because of traumatic neuropathy were enrolled, and the uncinate process was sampled during surgery. The controls were free of nasal symptoms, had no history of atopic disease, and had negative skin prick test results against common allergens. The demographic data from the CRS patients and the controls enrolled in this study are listed in Table 1. This study was approved by the Institutional Review Board, and informed consent was obtained from all subjects.
Each specimen was separated into 2 portions: one portion was immediately frozen in liquid nitrogen and stored at -80℃ for mRNA and protein examinations; the other portion was used for histological staining.
Human nasal tissues were stained with PAS, and the PAS-positive cells, which displayed a purple color, were counted via light microscopy (400×) and expressed as the percentage of the total epithelial cells (500 cells counted). IHC was performed as reported elsewhere,13 and the sections were stained using mouse monoclonal antibodies against the following proteins: FoxA2 (1:50; Millipore, Billerica, MA, USA), MUC5AC, and MUC5B (1:100; Santa Cruz Biotech, Santa Cruz, CA, USA). Then, the antibodies were detected via streptavidin-biotin-horseradish peroxidase complex formation. The immunostaining result was considered positive when brown cells appeared following reaction with the reagent 3', 3'-diaminobenzidine. Replacement of primary antibodies with isotype-matched IgG was used as a negative control. The sections were examined via light microscopy (400×), and the patterns of antibody staining were scored in a quantitative manner. The pattern of immunoreactivity was analyzed in the epithelium and subepithelial area. The number of immunoreactive cells (stained brown) was expressed as the percentage of the total cell number (500 cells counted). The immunoreactivity score was assessed by 2 independent observers in a blinded manner, and their results were averaged.
Total RNA was extracted from nasal tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Reverse transcription (RT) was performed, and cDNA was synthesized from 2 µg of total RNA using an oligo(dT)18 primer and M-MLV reverse transcriptase (TAKARA, Syuzou, Shiga, Japan) for quantitative PCR. We performed qRT-PCR using the ABI PRISM 7600 Detection System (Applied Biosystems, Foster City, CA, USA) and SYBR Premix Taq™ (TAKARA).14 The primer sequences for FoxA2, MUC5AC, MUC5B and β-actin were as follows: FoxA2 forward: 5'- TCT TAA GAA GAC GAC GGC TTC AG -3'; FoxA2 reverse: 5'- TTG CTC TCT CAC TTG TCC TCG AT -3'; MUC5AC forward: 5'- TCA GCC CCG AGT TCA AGG -3'; MUC5AC reverse: 5'- TTC CCA AAC TCC AGC ACG TC -3'; MUC5B forward: 5'- AGT CCA TTT GCT GAC CCC AC -3'; MUC5B reverse: 5'- GGA TGG TCG TGT TGA TGC G -3'; β-actin forward: 5'- AAG ATG ACC CAG ATC ATG TTT GAG ACC -3'; and β-actin reverse: 5'- AGC CAG GTC CAG ACG CAG GAT -3'. The mean value of the replicates for each sample was calculated and expressed as the cycle threshold (Ct). The mRNA expression level was calculated as the difference (ΔCt) between the Ct value of the target gene and the Ct value of β-actin. The fold change in the target gene mRNA levels was calculated as 2-ΔΔCt.
Tissue lysates were prepared by adding 1 mL of PBS containing a protease inhibitor cocktail (Keygentec, Nanjing, China) to every 100 mg of tissue. The protein concentration was determined using the BCA method. The level of secreted MUC5AC in the supernatants was measured via a sandwich ELISA developed in our laboratory using anti-MUC5AC and anti-MUC5B antibodies (Santa Cruz Biotech) as we have described elsewhere. Briefly, 10 µL of the collected sample and 40 µL of diluent were added to 96-well plates precoated with the captured MUC5AC antibody (1:200) for the assay. After incubation and washing, the anti-MUC5AC and -MUC5B antibodies (1:200) were added to each well. After incubation and washing, 100 µL of horseradish peroxidase-conjugated goat anti-mouse IgG (1:10,000) was added to each well. The colorimetric reaction was developed using 3, 3', 5, 5'-tetramethylbenzidine peroxide solution and stopped using 1 mol/L H2SO4. The absorbance at 450 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA), and the optical density (OD) value at 450 nm was recorded. The amount of MUC5AC and MUC5B in each sample was normalized to the total protein in the tissue lysates and expressed as the OD value per mg of total tissue protein.
Nasal secretions were collected as we have reported elsewhere.15 Briefly, open-cell flexible polyurethane foam was cut by the local distributor into sampler cubes of 30×20×10 mm3. The fluid retention capacity of each sampler was 3 mL. The samplers were placed in each nasal cavity posterior to the mucocutaneous junction under direct visualization and left in place for 10 minutes. Following removal of the samplers, the fluid was extracted from each sampler via centrifugation. The protein levels of IFN-γ, IL-4, IL-5, IL-6, and IL-17A in the nasal secretions were quantified using a FlowCytomix™ kit (eBioscience, San Diego, CA, USA). The detection limits for IFN-γ, IL-4, IL-5, IL-6, and IL-17A were 1.6, 3.0, 1.0, 1.5, and 1.2 pg/mL, respectively.
For in vitro cell isolation and culture, primary polyp-derived epithelial cells (PECs) were randomly collected from 4 CRSwNP patients via enzymatic digestion as described elsewhere.14 The collected PECs were cultured as submersion cultures in BEGM medium (Lonza, Walkersville, MD, USA) until passaging. At 80%-90% confluency, the cells were stimulated with recombinant human IL-6 (10 ng/mL; R&D Systems, Minneapolis, MN,USA) for different periods. To screen gene expression levels, genome-wide gene expression analysis was performed on the IL-6-stimulated PECs (10 ng/mL for 24 hours) using the Human 12×135 K Gene Expression Array (Catalog No. 05543789001, Roche NimbleGen, Inc., Madison, WI, USA) according to the manufacturer's protocol. To evaluate the effect of macrolide on IL-6-induced FoxA2 and MUC5AC expression, PECs were incubated in CAM (10 ng/mL; Sigma-Aldrich, St. Louis, MO, USA) for 12 or 24 hours in the presence of IL-6 (10 ng/mL). Then, cell pellets were collected for qRT-PCR or Western blot.
NCI-H292 cells (purchased from ATCC, MD, USA) were seeded in a 24-well plate and cultured in 2 mL of RPMI 1640 medium containing 10% FBS. The human FoxA2 expression plasmid (pGL-FoxA2) and the MUC5AC promoter-firefly luciferase reporter constructs were provided by Land Biotech (Guangzhou, China). All constructs were further confirmed by DNA sequencing. The transfection of NCI-H292 cells with the MUC5AC promoter-luciferase constructs, pGL-FoxA2 or FoxA2 siRNA (50 nmol/L) (Shanghai GenePharma Co., Ltd., Shanghai, China) or other control constructs was conducted using a Lipofectamine 2000-based gene transfer method (Invitrogen) according to the manufacturer's specifications. The cells were collected for further use after IL-6 treatment for 12 or 24 hours. For the luciferase activity assay, the cells were lysed in luciferase lysis buffer (25 mM Tris-phosphate (pH 7.8), 8 mM MgCl2, 1 mM DTT, 1% Triton X-100, and 15% glycerol), and a dual luciferase reporter assay kit (Promega, Madison, WI, USA) was used to analyze the firefly luciferase activity of the MUC5AC promoter and the Renilla luciferase activity of the internal control. For each transfection, the relative firefly luciferase activity level was normalized to the Renilla luciferase activity level.
Western blotting was performed as reported elsewhere.14 Briefly, total protein was extracted from the isolated cells in 100 µL of RIPA lysis buffer. The protein concentration in the supernatants was determined using the BCA method. Samples containing 20 µg of protein were boiled and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 8% Tris-glycine gels and electrophoretically transferred to a polyvinylidene fluoride membrane, which was incubated in 5% fat-free skim milk in Tris-buffered solution (TBS) containing 0.05% Tween-20 (1 hour at room temperature) and then incubated in mouse anti-human FoxA2 (Millipore) and GAPDH monoclonal antibodies (Santa Cruz Biotech) diluted 1:2,000 (overnight at 4℃). The membrane was washed and incubated in goat anti-mouse IRDye 800 and goat anti-rabbit Alexa Fluor 680 antibodies (Invitrogen) for 1 hour. Then, the membrane was washed 3 times with TBS-Tween and visualized using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). The membrane was scanned at 700 and 800 nm, and the results were analyzed using the Odyssey® software, v1.2.
The tissue data are expressed as the medians and interquartile ranges except for the additional note. These data were analyzed via the Kruskal-Wallis H-test and the nonparametric Mann-Whitney U test. For the in vitro experiments, the data were analyzed via one-way ANOVA and Student's t test. The correlation between different gene and protein expression levels in CRS patients was assessed using the Spearman rank correlation test. A P value of less than 0.05 was considered significant.
PAS staining indicated that mucin was extensively expressed in both the epithelium and gland cells in the human nasal mucosa in all analyzed cases. The cellular positivity of mucin was significantly higher in the epithelial and glandular mucous cells in both the CRSsNP and CRSwNP patients than in the normal controls (P<0.05, Fig. 1A and Table 2). In agreement with the PAS staining results, MUC5AC and MUC5B were detected in all analyzed cases. MUC5AC was highly expressed in the epithelial mucous cells and mildly expressed in the glandular mucous cells, whereas MUC5B was moderately expressed in the epithelial mucous cells and highly expressed in the glandular mucous cells. The cellular positivity of MUC5AC- and MUC5B-secreted cells significantly increased in both the CRSsNP and CRSwNP patients compared to the normal controls (P<0.05; Fig. 1B and C, and Table 2). No significant difference in MUC5AC or MUC5B immunoreactivity was observed between the CRSsNP and CRSwNP patients. FoxA2 was expressed by the epithelial and glandular mucous cells in the normal controls and localized to the cytoplasm and the nuclei of these cells. FoxA2 protein staining in the epithelial and glandular mucous cells significantly decreased in both the CRSwNP and CRSsNP patients compared to the normal controls (P<0.05, Fig 1D and Table 2). No significant difference in FoxA2 immunoreactivity was observed between the CRSsNP and CRSwNP patients.
As indicated by qRT-PCR analysis, the mRNA expression of FoxA2, MUC5AC, and MUC5B was detected in all analyzed cases. The relative mRNA levels of MUC5AC and MUC5B significantly increased in the CRSsNP and CRSwNP patients compared to the normal controls (P<0.05). Conversely, the relative FoxA2 mRNA level significantly decreased in the CRSsNP and CRSwNP patients compared to the normal controls (P<0.05, Fig. 2A-C). No difference in the FoxA2, MUC5AC or MUC5B mRNA levels was observed between the CRSsNP and CRSwNP patients. Consistently, the relative MUC5AC and MUC5B protein levels significantly increased in the CRSsNP and CRSwNP patients compared to the normal controls (P<0.05, Fig. 2D and E), whereas the relative FoxA2 protein level significantly decreased in the CRSsNP and CRSwNP patients compared to the normal controls (P<0.05, Fig. 2F and G). The expression level of FoxA2 significantly correlated with the expression levels of MUC5AC and MUC5B in CRS patients (r=-0.56, P<0.05, and r=-0.40, P<0.05, respectively).
The levels of IFN-γ, IL-4, IL-5, IL-6, and IL-17A in nasal secretions were significantly higher in both the CRSsNP and CRSwNP patients than in the normal controls (P<0.05, Fig. 3A-E). However, whereas the level of IFN-γ in the nasal secretions was significantly higher in the CRSsNP patients than in the CRSwNP patients (P<0.05, Fig. 3A), the levels of IL-4 and IL-5 in the nasal secretions were significantly lower in the CRSsNP patients than in the CRSwNP patients (P<0.05, Fig. 3B and C). No difference in the level of IL-6 or IL-17A was observed between the CRSsNP and CRSwNP patients (Fig. 3D and E). Based on Spearman rank correlation analysis, a significant correlation was observed between the relative FoxA2 expression level and the IL-6 concentration in the CRSsNP and CRSwNP patients (r=-0.46, P<0.01, Fig. 3F). However, no correlation was detected between the levels of the other 2 cytokines and the FoxA2 protein expression level in the CRS patients.
Next, we evaluated the regulatory effect of FoxA2 on MUC5AC production via in vitro stimulation. DNA microarray analysis of NCI-H292 cells revealed a series of up-regulated and down-regulated genes in NCI-H292 cells after stimulation with IL-6 (10 ng/mL) for 12 hours. Of these genes, FoxA2 was found to be significantly down-regulated (-2.45-fold, P<0.05, Fig. 4A). Consistently, FoxA2 mRNA expression was significantly and dose-dependently reduced in the cells stimulated with IL-6 for 12 hours compared to the unstimulated cells (P<0.05, Fig. 4C). FoxA2 protein expression was also significantly reduced in the cells stimulated with IL-6 for 24 hours (P<0.05, Fig. 4D). In contrast, the MUC5AC mRNA level was significantly and dose-dependently up-regulated after IL-6 stimulation (P<0.05, Fig. 4B). After transfection of the IL-6-stimulated NCI-H292 cells with pGL-FoxA2, the FoxA2 protein level was significantly increased compared to the control cells (P<0.05, Fig. 4E), and MUC5AC promoter activity, as suggested by the luciferase activity assay, was significantly inhibited (P<0.05, Fig. 4F). Moreover, the MUC5AC mRNA level was significantly down-regulated in the presence of the FoxA2 expression plasmid and was significantly upregulated in the presence of FoxA2 siRNA compared to the control treatment (P<0.05, Fig. 4G and H).
In the next experiment, we examined the mRNA and protein levels of FoxA2 in polyp tissues from 9 CRSwNP patients before and after the oral administration of CAM (250 mg, twice daily) for 2 consecutive weeks. Consequently, we found that the individual nasal symptom scores (rhinorrhea and nasal congestion) were significantly decreased after the oral administration of CAM (P<0.01, Fig. 5A and B). FoxA2 immunoreactivity and mRNA expression in these polyp tissues were significantly increased after oral administration of CAM (P<0.01, Fig. 5C and D). Accordingly, CAM (10 ng/mL) significantly attenuated IL-6-induced FoxA2 mRNA and protein suppression in cultured PECs in a dose-dependent manner (P<0.05, Fig. 5E-G), whereas the MUC5AC mRNA level was significantly reduced in response to CAM administration (P<0.05, data not shown).
This study demonstrated aberrant FoxA2 and mucin expression in inflamed nasal tissue from CRS patients, which was differentially modulated by proinflammatory cytokines and anti-inflammatory macrolides. The results of this study support an important role of FoxA2 in the negative regulation of mucin production in CRSsNP and CRSwNP patients, which expands our understanding of the pathophysiology of CRS.
The winged helix factor FoxA2 is an essential member of the transcription factor network that represents a common signaling node for multiple goblet cell differentiation signaling pathways, and FoxA2 is a known to suppress mucus hypersecretion.16 Yu et al.17 have demonstrated that FoxA2 significantly inhibits the activity of a MUC5AC-luciferase construct in a dose-dependent manner in 16HBE cells. Wan et al.6 have reported that the conditional deletion of FoxA2 in mouse airway epithelial cells leads to a large increase in the number of goblet cells and that the nuclear expression of the FoxA2 protein is reduced in the goblet cells of IL-13-overexpressing mice. In transgenic mice that overexpress IL-1β, decreased expression of FoxA2 is associated with increased numbers of MUC5AC-positive goblet cells.1819 Therefore, FoxA2 plays an important role in protecting airway epithelial cells against excessive mucin production, and the regulation of FoxA2 may be considered a key factor in the induction of mucus hyperserection.2021 Although many studies have reported decreased FoxA2 expression in the airway epithelium, this is the first report to investigate FoxA2 expression in inflamed nasal tissues of CRS patients. Using IHC staining, qRT-PCR, and Western blot, we detected decreased mRNA and protein levels of FoxA2 in the CRSsNP and CRSwNP patients compared to the normal controls. Whereas the FoxA2 mRNA and protein levels were significantly down-regulated, the MUC5AC and MUC5B mRNA levels were significantly up-regulated in the nasal tissues of both CRSsNP and CRSwNP patients. The level of FoxA2 significantly correlated with the expression levels of MUC5AC and MUC5B in these CRS patients. Our results strongly suggest that the down-regulation of FoxA2 in CRS patients contributes to the associated increase in the MUC5AC and MUC5B levels. Thus, FoxA2 and its upstream signaling pathway may serve as an important regulator of the inflammatory and repair processes that are associated with nasal mucin hypersecretion.
Recent studies have shown that both IL-13 and EGFR signaling are necessary for mucus production and that these signaling pathways differentially regulate various mucus-producing cell types.22 In our study, increased levels of IFN-γ, IL-4, IL-5, IL-6, and IL-17A, but not IL-13 (data not shown), were observed in the nasal secretions of CRS patients compared to those of normal controls. Based on the significant differences in the IFN-γ and IL-4 levels between the CRSsNP and CRSwNP patients, it is likely that Th1-related cytokine IFN-γ and Th2-related cytokine IL-4 did not strongly regulate MUC5AC or MUC5B expression in these CRS patients. However, we observed an increased level of IL-6 in both CRS subpopulations (no difference between the CRSsNP and CRSwNP patients) and found the IL-6 level was negatively associated with FoxA2 production, suggesting that the IL-6 signaling pathway might play a more important role than other cytokine-mediated pathways in FoxA2 suppression and mucin production in these CRS patients. Previously, IL-6 has been demonstrated to regulate FoxA2 expression and mucin production in epithelial cells. For example, Chen et al.6 have reported that IL-17A stimulates airway mucin gene expression via an IL-6-mediated paracrine/autocrine feedback loop, suggesting that IL-6 is critical for mucus hypersecretion in respiratory disease. Verschuur et al.23 have found that FoxA2 site that is located immediately upstream of previously identified IL-6-responsive sequences. Currently, accumulating evidence indicates that the IL-6 signaling pathway plays a crucial role in the development and persistence of CRS.91011121324 To evaluate whether IL-6 promotes FoxA2 suppression and mucin upregulation, we further examined the effects of IL-6 on FoxA2 and MUC5AC expression in vitro. As expected, IL-6 was found to significantly inhibit FoxA2 mRNA expression in a dose-dependent manner in vitro, whereas the MUC5AC mRNA expression level was increased after IL-6 stimulation. Moreover, using a luciferase activity assay, we found that FoxA2 regulates MUC5AC promoter activity. After transfection of NCI-H292 cells with pGL-FoxA2, the FoxA2 mRNA and protein expression levels were significantly increased compared to the control treatment. Consistently, pGL-FoxA2 and FoxA2 siRNA differentially regulated IL-6-stimulated MUC5AC up-regulation in vitro. These findings indicate that FoxA2 plays a critical role in modulating IL-6-induced mucin hypersecretion.
Recently, macrolide therapy has been suggested as an alternative treatment for CRS and other respiratory diseases.25 A growing body of evidence suggests that several macrolides, including azithromycin and CAM, are effective for the modulation of mucus synthesis and secretion.26 Molecular mechanisms underlying the effects of macrolide administration are not completely understood. It has been proposed that the therapeutic effects of macrolides are due to anti-inflammatory rather than anti-microbial activities and involve interactions with phospholipids and Erk1/2, which lead to the modulation of the transcription factors AP-1 and NFκB, inflammatory cytokines, and mucin release,27282930 although the mode of action of macrolides is likely complex. In our study, we provided evidence that CAM administration increases FoxA2 production and inhibits MUC5AC expression in CRS patients. These results are of significant interest because, to our knowledge, this is the first study to demonstrate that the alteration of FoxA2 expression after IL-6 stimulation was strongly associated with mucin overproduction in CRS patients. Because it has been shown that intranasal steroids decrease eosinophils but not mucin expression in nasal polyps31 and because IL-13-induced MUC5AC production and goblet cell differentiation is steroid-resistant in human airway cells,32 our findings might be beneficial for designing alternative treatment strategies for mucin hypersecretion in CRS patients.
In conclusion, we have shown that the IL-6 signaling pathway is involved in the suppression of FoxA2 expression, which subsequently promotes excessive mucin production in CRSsNP and CRSwNP patients. Our findings imply that targeting FoxA2 may serve as a selective therapeutic strategy for the treatment of mucin hypersecretion in both CRSsNP and CRSwNP patients.
Figures and Tables
Fig. 1
Immunoreactivity of MUC5AC, MUC5B, and FoxA2 in the nasal tissues from the CRSsNP and CRSwNP patients and the normal controls. PAS staining shows that mucin is highly expressed in the epithelial and glandular mucous cells of CRS patients (A); IHC staining shows that MUC5AC is highly expressed in the epithelial mucous cells and mildly expressed in the glandular mucous cells (B) and that MUC5B is moderately expressed in the epithelial mucous cells and highly expressed in the glandular mucous cells (C). Both MUC5AC and MUC5B are mildly expressed in the normal controls. FoxA2 is highly expressed in the epithelial and glandular mucous cells of the normal controls and mildly detected in the cytoplasm and the nuclei of these cell types (D) (magnification, 200×).
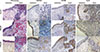
Fig. 2
The mRNA and protein levels of MUC5AC, MUC5B, and FoxA2 in the nasal tissues from the CRSsNP and CRSwNP patients and the normal controls. The relative mRNA levels of MUC5AC (A) and MUC5B (B) increase in the CRSsNP and CRSwNP patients compared to the normal controls. The relative FoxA2 mRNA level decreases in the CRSsNP and CRSwNP patients compared to the normal controls (C). The protein levels of MUC5AC (D) and MUC5B (E) significantly increase in the CRSsNP and CRSwNP patients compared to the normal controls. A representative Western blot of FoxA2 in the CRSsNP and CRSwNP patients and the normal controls is shown (F). The relative FoxA2 protein level significantly decreases in the CRSsNP and CRSwNP patients compared to the normal controls (G). *P<0.05.

Fig. 3
The levels of IFN-γ, IL-4, IL-5, IL-6, and IL-17A in the nasal secretions of the CRSsNP and CRSwNP patients and the normal controls. The levels of IFN-γ (A), IL-4 (B), IL-5(C), IL-6 (D), and IL-17A (E) in the nasal secretions are significantly higher in both the CRSsNP and CRSwNP patients than in the normal controls. The relative FoxA2 protein level in the inflamed nasal tissues is negatively associated with the levels of IL-6 in the nasal secretions of the CRSsNP and CRSwNP patients. *P<0.05; **P<0.01.

Fig. 4
FoxA2 negatively regulates IL-6-induced MUC5AC production in NCI-H292 cells. DNA microarray analysis of NCI-H292 cells in response to IL-6 (10 ng/mL) stimulation. A heatmap of the genes that were up-regulated or down-regulated in the IL-6-induced NCI-H292 cells is shown (A). IL-6 (0-20 ng/mL)-induced MUC5AC mRNA expression in a dose-dependent manner (B). IL-6 (0-20 ng/mL) inhibits FoxA2 mRNA and protein expression in a dose-dependent manner (C, D). Representative Western blot for FoxA2 in NCI-H292 cells after transfection with the FoxA2 expression plasmid (E). Transfection with the FoxA2 expression plasmid significantly inhibits MUC5AC promoter activity in the presence of IL-6, as suggested by a luciferase activity assay (F). Transfection with the FoxA2 expression plasmid significantly inhibits IL-6-induced MUC5AC production (G). Transfection with FoxA2 siRNA significantly increases IL-6-induced MUC5AC production (H). The data are expressed as the means (SEM) of the 3 independent experiments. *P<0.05.

Fig. 5
CAM promotes FoxA2 expression in CRSwNP patients in vivo and in vitro. Individual nasal symptom scores (rhinorrhea and nasal congestion) before and after oral administration of CAM (250 mg, twice daily) for 2 weeks (A, B). Representative immunoreactivity of FoxA2 and MUC5AC in polyp tissues before and after oral administration of CAM for 2 weeks (C). FoxA2 mRNA expression in polyp tissues before and after oral administration of CAM for 2 weeks (D). CAM (10 ng/mL) significantly attenuates the IL-6-induced suppression of FoxA2 mRNA and protein expression in PECs (E-G). The in vitro data are expressed as the means (SEM) of the 3 independent experiments. *P<0.05; **P<0.01.

Table 1
Clinical characteristics of the CRS patients and the normal control subjects
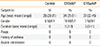
Table 2
Immunoreactivity of MUC5AC, MUC5B and FoxA2 in the nasal mucosa of the CRS# patients and the normal control subjects

ACKNOWLEDGMENTS
This study was supported by the National Natural Science Grants of China (No. 81271054, 81371074, 81470673) and grants from the Ministry of Hygiene (No. 201202005) and the Ministry of Education (No. 20130171110064). The authors have declared that they have no conflict of interest.
References
1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.
2. Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007; 117:932–938.
3. Ding GQ, Zheng CQ. The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2007; 21:359–366.
4. Kim DH, Chu HS, Lee JY, Hwang SJ, Lee SH, Lee HM. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004; 130:747–752.
5. Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans. 2009; 37:877–881.
6. Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004; 131:953–964.
7. McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005; 102:9589–9594.
8. Mitsuyama K, Sata M, Rose-John S. Interleukin-6 trans-signaling in inflammatory bowel disease. Cytokine Growth Factor Rev. 2006; 17:451–461.
9. Lennard CM, Mann EA, Sun LL, Chang AS, Bolger WE. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. 2000; 14:367–373.
10. Bradley DT, Kountakis SE. Role of interleukins and transforming growth factor-beta in chronic rhinosinusitis and nasal polyposis. Laryngoscope. 2005; 115:684–686.
11. Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003; 278:17036–17043.
12. Xu R, Xu G, Shi J, Wen W. A correlative study of NF-kappaB activity and cytokines expression in human chronic nasal sinusitis. J Laryngol Otol. 2007; 121:644–649.
13. Xu G, Xia JH, Zhou H, Yu CZ, Zhang Y, Zuo KJ, et al. Interleukin-6 is essential for Staphylococcal exotoxin B-induced T regulatory cell insufficiency in nasal polyps. Clin Exp Allergy. 2009; 39:829–837.
14. Liu WL, Zhang H, Zheng Y, Wang HT, Chen FH, Xu L, et al. Expression and regulation of osteopontin in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2015; 45:414–422.
15. Luo Q, Chen F, Liu W, Li Z, Xu R, Fan Y, et al. Evaluation of long-term clarithromycin treatment in adult Chinese Patients with chronic rhinosinusitis without nasal polyps. ORL J Otorhinolaryngol Relat Spec. 2011; 73:206–211.
16. Lai HY, Rogers DF. Mucus hypersecretion in asthma: intracellular signalling pathways as targets for pharmacotherapy. Curr Opin Allergy Clin Immunol. 2010; 10:67–76.
17. Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes. 2010; 17:83–92.
18. Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L420–L427.
19. Gray T, Nettesheim P, Loftin C, Koo JS, Bonner J, Peddada S, et al. Interleukin-1beta-induced mucin production in human airway epithelium is mediated by cyclooxygenase-2, prostaglandin E2 receptors, and cyclic AMP-protein kinase A signaling. Mol Pharmacol. 2004; 66:337–346.
20. Hao Y, Kuang Z, Xu Y, Walling BE, Lau GW. Pyocyanin-induced mucin production is associated with redox modification of FOXA2. Respir Res. 2013; 14:82.
21. Hao Y, Kuang Z, Walling BE, Bhatia S, Sivaguru M, Chen Y, et al. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell Microbiol. 2012; 14:401–415.
22. Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007; 36:244–253.
23. Verschuur M, de Jong M, Felida L, de Maat MP, Vos HL. A hepatocyte nuclear factor-3 site in the fibrinogen beta promoter is important for interleukin 6-induced expression, and its activity is influenced by the adjacent -148C/T polymorphism. J Biol Chem. 2005; 280:16763–16771.
24. Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010; 125:397–403.e10.
25. Cameron EJ, McSharry C, Chaudhuri R, Farrow S, Thomson NC. Long-term macrolide treatment of chronic inflammatory airway diseases: risks, benefits and future developments. Clin Exp Allergy. 2012; 42:1302–1312.
26. Shimizu T, Shimizu S, Hattori R, Gabazza EC, Majima Y. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. Am J Respir Crit Care Med. 2003; 168:581–587.
27. Araki N, Yanagihara K, Morinaga Y, Yamada K, Nakamura S, Yamada Y, et al. Azithromycin inhibits nontypeable Haemophilus influenzae-induced MUC5AC expression and secretion via inhibition of activator protein-1 in human airway epithelial cells. Eur J Pharmacol. 2010; 644:209–214.
28. Ou XM, Feng YL, Wen FQ, Wang K, Yang J, Deng ZP, et al. Macrolides attenuate mucus hypersecretion in rat airways through inactivation of NF-kappaB. Respirology. 2008; 13:63–72.
29. Tanabe T, Kanoh S, Tsushima K, Yamazaki Y, Kubo K, Rubin BK. Clarithromycin inhibits interleukin-13-induced goblet cell hyperplasia in human airway cells. Am J Respir Cell Mol Biol. 2011; 45:1075–1083.
30. Kanai K, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase production from nasal fibroblasts by macrolide antibiotics in vitro. Eur Respir J. 2004; 23:671–678.
31. Burgel PR, Cardell LO, Ueki IF, Nadel JA. Intranasal steroids decrease eosinophils but not mucin expression in nasal polyps. Eur Respir J. 2004; 24:594–600.
32. Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy. 2011; 41:1747–1756.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download